Abstract
The mechanical properties of deep fasciae strongly affect muscular actions, development of pathologies, such as acute and chronic compartment syndromes, and the choice of the various fascial flaps. Actually, a clear knowledge of the mechanical characterization of these tissues still lacks. This study focuses attention on experimental tests of different regions of human crural fascia taken from an adult frozen donor. Tensile tests along proximal–distal and medial–lateral direction at a strain rate of 120 %/s were performed at the purpose of evaluating elastic properties. Viscous phenomena were investigated by applying incremental relaxation tests at total strain of 7, 9 and 11 % and observing stress decay for a time interval of 240 s. The elastic response showed that the fascia in the anterior compartment is stiffer than in the posterior compartment, both along the proximal–distal and medial–lateral directions. This result can explain why the compartment syndromes are more frequent in this compartment with respect to posterior one. Furthermore, the fascia is stiffer along the proximal–distal than along medial–lateral direction. This means that the crural fascia can adapt to the muscular variation of volume in a transversal direction, while along the main axis it could be considered as a structure that contributes to transmitting the muscular forces at a distance and connecting the different segments of the limb. The stress relaxation tests showed that the crural fascia needs 120 s to decrease stress of 40 %, suggesting a similar time also in the living so that the static stretching could have an effect on the fascia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
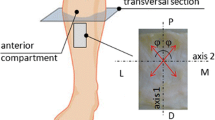
Crural fascia is a dense connective tissue that envelops and separates portions of the muscles of the leg into three different compartments: the anterior, the posterior and the lateral compartment (Fig. 1). It is suggested that the crural fascia executes integration and forces transmission between these compartments and plays an important role in the biomechanical behavior of the leg [2, 12]. Besides, the crural fascia plays a fundamental role in the etiology of the acute and chronic exertional compartment syndrome (CECS) that is a condition in which chronic increased pressure within a closed osteofascial compartment compromises blood flow in the muscles and nerves within that compartment, resulting in pain and disability [5, 7, 14]. In particular, the CECS is frequent in athletes that occur in repetitive loading, long-distance runners, skiers, basketball and soccer players, but no standard diagnostic procedure is currently universally accepted and what contributes to the increased intramuscular pressure remains unknown. It is probably that in these subjects the aponeurotic fasciae are not able to adapt to the variations of muscular volume during sport activities, but Dahl et al. [5] demonstrated that differences in fascial stiffness or thickness in patients with CECS compared with healthy individuals do not exist. Besides, it is not clear why it is more frequent in the anterior compartment with respect to the posterior and the lateral one.
By now the studies about crural fascia, and deep fascia in general, have mainly considered its anatomical and histological conformation leading to the evidence that it is composed of subsequent layers of dense connective tissues, separated by a layer of loose connective tissue [1, 9–12]. With regard to crural fascia, previous studies report that each layer shows a mean thickness of 277.8 μm (standard deviation, SD = 86.61 μm) while the layer of loose connective tissue has a mean thickness of 43 μm (SD = 12 μm) [11]. Furthermore, it has been observed that each layer of dense connective tissue shows the presence of collagen fibers oriented according to a specific direction. The fibers of a layer form angles of about 80° with the fibers of the adjacent and, globally, there is a symmetrical disposition with respect to the proximal–distal direction, with angles of about 40° [11]. Actually, there are very few studies at disposal about fascia mechanical characterization [7, 14] and an in-depth investigation is necessary about fascia stress–strain behavior, hysteresis characteristics in loading–unloading cycle and degree of homogeneity in mechanical responses according to the anatomical location and direction of force applied.
Therefore, the present work intends to be a preliminary assessment of the mechanical properties of the crural fascia, including the evaluation of the variability of the properties as a function of the sample location to get a better understanding of the compartment syndromes. Starting from the above-mentioned anatomical and morphological evidences, it was possible to set different experimental protocols to separately investigate the elastic and the viscous response, leading to an initial assessment of the mechanical behavior of the human crural fascia.
Materials and methods
Samples preparation and experimental setup
This study analyses different regions of crural fascia taken from the same frozen adult donor (male, age 67, weight 74.8 kg and height 165.1 cm). Samples of crural fascia were dissected from the anterior compartment of the right and the left legs and from the posterior compartment of the right leg, where the deep fascia was easily separable from both the superficial fascia and the muscular plane. Samples originating from the left posterior compartment were damaged throughout dissection, because of the presence of many adherences; as a consequence, it was excluded from the experiment. After the dissection, the samples were frozen and kept at a temperature of −20 °C until 20 min before the experimental test when they were immerged in a saline solution (phosphate buffered solution, PBS) at a temperature of 20 °C. This solution has the double effect of speeding up the unfrozen process and preserving samples hydration.
The samples of crural fascia were cut into smaller specimens along the proximal–distal and medial–lateral directions. A total of 17 specimens originated from anterior compartments and nine specimens from posterior compartments. The detail of the specimens within each sample is reported in Table 1. Each specimen was identified by means of an alphanumeric code and its position was kept on a map of the original piece of tissue. Typical mapping of the sample is shown in Fig. 2 for the right posterior compartment. The following nomenclature identifies the samples: R/L = right/left leg, A/P = anterior/posterior compartment, sample number, L/T = longitudinal/transversal direction. The term longitudinal refers to a sample cut along the proximal-distal direction and the term transversal to a sample cut along the medial-lateral direction.
Samples location in the posterior compartment (a) longitudinal samples are cut along the distal–proximal direction, while transversal samples follow the medial–lateral orientation (RP = right posterior, L/T = longitudinal/transversal). The arrows in b represent the main collagen fibers orientations in the tissue
The specimens were cut with rectangular shape, length about 25–30 mm and width about 3 mm. The effective dimensions of the specimens were evaluated through image analysis of pictures taken with a digital camera. The mean thickness and SD of the specimens as a function of the region are reported in Table 1.
All the mechanical tests were developed with the Planar Biaxial TestBench Test Instrument (Bose® Electro-Force, USA). To avoid any slipping of the specimens during the test, extremities were fixed to the grips of the apparatus by means of patches of Velcro. The specimens were also marked on their thickness in the proximity of the grip to evaluate any possible slipping between grip and specimen during each test. The instrument was configured to impose uni-axial loading. The free region of samples between the grips was 10 mm length (‘gauge length’), therefore having an aspect ratio (length/width) of about 3 to 1. All the tests were developed with precision of 0.001 mm for displacement and 0.2 N for load. The specimens were kept in hydrated conditions throughout the testing time by pipetting with saline solution.
Mechanical experimental protocol
With the aim of evaluating separately the elastic instantaneous response and the time-dependent response due to viscous phenomena, two different kinds of experimental protocols were designed and performed. All the mechanical tests, of both protocols, start with ten cycles of sinusoidal preconditioning in the nominal strain range of 0–5 % at the frequency of 1 Hz followed by a rest period of 60 s to obtain a stable mechanical response in the preliminary phase [3, 4, 6, 8, 13].
Right leg tissues were tested to investigate the viscous time-dependent phenomena by means of three consecutive stress relaxation tests at nominal strain of 7, 9 and 11 % respectively, lasting 240 s. At the end of each relaxation time interval, the specimen was unloaded in 30 s. Each test was separated from the next one by a rest period to allow the complete development of viscous phenomena induced by the unloading phase. The strain level was applied at strain rate of 120 %/s to avoid the development of viscous phenomena in the lengthening ramp preceding the stress relaxation phase. This was supported by the instrument performance without introducing any distortion in the recorded signal.
The tissue taken from the left leg was used for the evaluation of the elastic properties of the fascia. After the initial preconditioning phase, three sets of five loading–unloading cycles each were applied at the increasing nominal strain of 7, 9 and 11 % respectively. Loading–unloading cycles were applied at strain rate of 120 %/s and were separated one from the other by a rest period to allow the development of viscous phenomena. Five cycles per set were chosen on the basis of experimental evidences that showed a stabilization of the mechanical response of the tissue after such a number of loading–unloading cycles.
All the mechanical tests, both on the right and left leg specimens ended with a ramp-to-failure consisting of an elongation up to a maximum strain of 120 % at the strain rate of 120 %/s to evaluate the ultimate stress and the failure strain, if any.
The load–displacement data recorded were processed to calculate stress–strain values. The nominal strain was calculated as the current lengthening of the sample divided by its initial grip-to-grip length. True stress (Cauchy stress) was computed as the force recorded by the load cell divided by the current value of the deformed cross-sectional area of the sample. The value of the deformed area was calculated by dividing the value of the undeformed cross-sectional area by the strain value, exploiting the volume conservation constraint that can be assumed because of the liquid content of the tissue. To compare experimental data with outcomes of previous works from the literature the response of the tissue has been also evaluated in terms of experimental data obtained also in terms of material stiffness, which is calculated as the slope of the quasi-linear portion of the stress strain curve of the failure tests (Fig. 3). Another parameter was also considered that gives a measure of stiffness per unit width of the specimen. It was computed as the product of the material stiffness and the initial sample thickness.
A univariate statistical analysis (ANOVA) was performed on the stress relaxation values at the different time instants of 25, 135 and 235 s making a comparison among the different levels of strain applied (7, 9 and 11 %). The same statistical tests were performed on stiffness values of longitudinal and transversal specimens of both the anterior and the posterior compartments to analyze differences due to anisotropic structural conformation.
Results
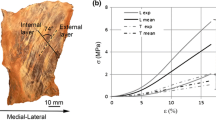
The mechanical response of the crural fascia shows typical behavior of soft connective tissues. Tensile tests (Fig. 3a) reveal a typical stiffening behavior that can be evaluated by the increasing slope of the stress–strain curve, up to the damage region where the increase in strain causes the progressive failure of the tissue. Indeed, the crural fascia shows to have viscoelastic behavior in a physiological range of strain. The viscoelastic characteristics of the tissue can be seen for example in the existence of hysteresis phenomena (Fig. 3b).
Comparing the average mechanical response to uni-axial tensile tests between longitudinal and transversal samples taken from the anterior (Fig. 4a) and posterior (Fig. 4b) compartment, it is shown that the samples in longitudinal direction are stiffer than the samples in transversal direction. In fact, experimental data show that at the same level of strain the force per unit of width in the longitudinal samples is always greater than in the transversal samples. As a way of example for strain of 10 %, the average force per unit of width in the longitudinal samples is about 7.5 N/mm while in the transversal samples the same is about 2.1 N/mm. The highlighted anisotropic characteristics, due to the collagen fibers orientation, are also underlined by the computed structural stiffness values that define the stiffness per unit width (Table 2).
When considering the anterior and posterior longitudinal samples, it is evident a greater stiffness of longitudinal anterior samples with respect to longitudinal posterior samples (Fig. 5a). At the level of 10 % of strain, average force per unit of width in the longitudinal anterior samples is about 3.5 N/mm, while in the posterior longitudinal samples is about 2.5 N/mm. This difference is not so pronounced if considering transversal specimens (Fig. 5b).
Comparison of the average mechanical behavior to uni-axial tensile tests on longitudinal (a) and transversal (b) samples originating from both the anterior and the posterior compartments (L/T = longitudinal/transversal, A/P = anterior/posterior). The curves are expressed in terms of force per unit of sample width vs. nominal strain
The results of the statistical analysis show a significant difference in stiffness values (p = 0.0001) with regard to the comparison between longitudinal and transversal specimens originating from the anterior compartment. The same test performed on specimens of the posterior compartment supplies a higher p value (0.057) that is very close to the threshold value. This is probably due to the limited number of available specimens. The computed structural stiffness assumes values that are close to experimental data previously provided by Hurschler et al. [7], who reported an average structural stiffness of 50.9 N/mm (SD = 33 N/mm) in longitudinal direction.
Previous curves are limited to maximum strain of about 24 %, value at which the weakest specimen showed the initial failure. Defining this characteristic point as peak strain, it was found that the average value was about 27 % for the specimens of the anterior compartment, and 27.5 % for the specimens of the posterior compartment.
The viscoelastic response of the tissue during relaxation tests is evaluated in terms of stress at current time divided by the peak stress value at the beginning of the relaxation process. It is shown that in longitudinal anterior samples (Fig. 6a), the stress at 60 s is about the 68 % of the peak stress, while in the following 120 s the stress is reduced only to the value of 63 % of the peak stress. Similar behavior is found for the longitudinal samples of the posterior region (Fig. 6b). At 60 s of relaxation time, the stress is about 73 % of the peak stress, while in the following 120 s the value is reduced to about 68 % of the peak stress. Average stress decay shows that stress relaxation is similar in longitudinal and transversal samples taken from the anterior region (Fig. 7). The average stress in the transversal samples at 60 s of the relaxation time is about 78 % of the peak stress, while in the following 120 s it evaluated a reduction to 73 % of the peak stress.
Average stress relaxation and SD vs. time for the longitudinal samples of the anterior (a) and posterior (b) compartments at imposed strain of 7, 9 and 11 %. The stress relaxation is expressed in terms of current stress divided by the stress value at the initial time instant of the relaxation process
The results of the statistical analysis, which compare stress relaxation values among the different levels of strain applied (7, 9 and 11 %), shows significant differences at the considered time instants between two clusters: the anterior samples tested at 7 % of strain applied and the posterior samples tested at a strain level of 11 %. All the other possible comparisons do not underline any statistical significant difference. Further, performing the same tests separately for the anterior and the posterior compartments, it is not found any significant difference between the considered groups (p values >0.05).
Discussion
The study of the elastic properties highlighted the different mechanical behavior among the specimens considered, depending on the strain level imposed, on the anatomical location of the sample and on collagen fibers arrangement and orientation.
The curves reported from the ramp-to-failure tests, limiting the representation to a strain in the physiological range, show tissue anisotropy due to the specific collagen fibers orientation and show the great stiffness of longitudinal samples with respect to transversal samples, both in anterior and posterior compartments (Fig. 4). In general, samples taken from anterior regions are stiffer than samples originating from the posterior compartment (Fig. 5). This finding well corresponds to the evidence that derives from the clinical practice considering, for example, the fact that the anterior compartment syndrome is more frequent than the posterior one. The evaluation of the anisotropic characteristics in fascial tissues can assume relevance also for those fasciae that are adopted in surgical reconstruction. At this purpose, surgeons using fasciae as flaps should contemplate these features because flaps could have a different mechanical interaction with surrounding tissues according to their placement and the orientation of the collagen fibers with respect to loading directions.
These preliminary results confirm the idea that the structural conformation of the crural fascia well adapts to muscles contraction and local volume variation given by different physical conditions. In fact the adaptation to muscle shape largely involves the lengthening of the crural fascia along the transversal direction. The greater stiffness of the fascia along the longitudinal axis of the limb is in agreement with the capability of force transmission in the connections of the knee and the ankle.
With regard to the viscous phenomena, it was observed that the percentage of the relaxed stress scarcely depends on the strain level imposed, on the anatomical location of the sample and on collagen fibers disposition and orientation, showing a certain degree of homogeneity in experimental outcomes and allowing the assumption of a linear process (Figs. 6, 7). The closeness of the results is underlined by the average normalized stress relaxation values and corresponding SD reported in Table 3. These values are referred to different levels of strain applied in the considered time instants. This evidence is also supported by statistical analysis results performed separately for the anterior and posterior compartments. The outcomes do not show any significant difference between stress relaxation values if considering different levels of strain applied. If comparing the anterior and the posterior regions after 235 s, it is possible to underline a higher percentage of relaxed stress in the anterior compartment. The trend of the stress relaxation is characterised by a relevant decrease of the stress values during the initial portion of experimental tests. This behavior is typically associated with a rearrangement of the structural components of the tissue and with the migration of the liquid phases throughout the time, leading to stress decay as macroscopic effect.
In previous researches, the stress relaxation phenomenon was monitored in 120 s that were considered a sufficient time interval to reach the steady state [7, 14]. In the present study it was decided to adopt longer observation time intervals, of 240 s, to evaluate any development of viscous phenomena. With regard to the average data deduced from the longitudinal specimens, it was possible to compute a stress relaxation of about 32 % within the first 120 s, that successively reaches the value of about 37 % after 240 s. Taking into consideration the mathematical extrapolation from the stress decay data, it was possible to estimate the stress after a time interval of 500 s. This shows that relaxed stress does not change significantly, reaching the value of 37.4 % at 500 s. Therefore, time intervals of 240 s can be considered long enough for the evaluation of stress relaxation. Since this result comes from a numerical extrapolation, it has to be verified experimentally. Finally, the stress relaxation curves confirm that about the 90 % of this phenomenon takes place in the first minute after the application of the strain. Similar considerations could be made for the stress relaxation phenomena found in the transversal specimens. In the latter case, the stress relaxation is also developed for about 90 % in the first minute of the process, while the average relaxed stress is about 22 % at 120 s and about 26 % at 240 s. These data could suggest that the maintenance of a static stretching position for about 1 min is enough to let the fasciae reach a new balance. Further time cannot modify significantly the fascial tension.
Conclusion
The results of the present work provide a valid preliminary quantification of the mechanical behavior of the crural fascia tissue, investigated through different experimental tests. In particular, the main mechanical features of the crural fascia are characterized by anisotropy, highly non-linear stress–strain relationship and viscoelasticity. When it was possible to compare present data with mechanical characteristics of the fascia from other works, a good agreement was found.
The mechanical characterization of the crural fascia could represent a rational basis for the investigation of several aspects pertaining to physiology or pathological conditions due to different etiology. Furthermore, the collected experimental data will help in developing a constitutive model of the crural fascia for in silico analyses of the biomechanical behavior under different conditions. This could represent a powerful method for an accurate interpretation of aspects that are of peculiar interest in clinical practice.
A limit of the proposed investigation consists in the fact that all the tissues tested were taken from the same donor. However, it is pointed out that experimental findings are in agreement with previous literature remarks. To quantify the inter-individual variability of the phenomena taken into account and to confirm the findings of the present work, it will be necessary to extend this study to a great number of subjects, as already in progress. The extension of the study will allow further comparisons making possible to evaluate aspects related to other important factors as, for example, the age, the gender or the body side of the subjects.
References
Benettazzo L, Bizzego A, De Caro R, Frigo G, Guidolin D, Stecco C (2011) 3D reconstruction of the crural and thoracolumbar fasciae. Surg Radiol Anat 33:855–862. doi:10.1007/s00276-010-0757-7
Benjamin M (2009) The fascia of the limbs and back—a review. J Anat 214:1–18. doi:10.1111/j.1469-7580.2008.01011.x
Carew EO, Barber JE, Vesely I (2000) Role of preconditioning and recovery time in repeated testing of aortic valve tissues: validation through quasilinear viscoelastic theory. Ann Biomed Eng 28:1093–1100. doi:10.1114/1.1310221
Cheng S, Clarke EC, Bilston LE (2009) The effects of preconditioning strain on measured tissues properties. J Biomech 42:1360–1362. doi:10.1016/j.jbiomech.2009.03.023
Dhal M, Hansen P, Stal P, Edmundsson D, Magnusson SP (2011) Stiffness and thickness of fascia do not explain chronic exertional compartment syndrome. Clin Orthop Relat Res 469:3495–3500. doi:10.1007/s11999-011-2073-x
Einat R, Yoram L (2010) Recruitment viscoelasticity of the tendon. J Biomed Eng 43:3177–3182. doi:10.1115/1.3212107
Hurschler C, Vanderby R, Martinez DA, Vailas AC, Turnipseed WD (1994) Mechanical and biomechanical analyses of tibial compartment fascia in chronic compartment syndrome. Ann Biomed Eng 22:272–279. doi:10.1007/BF02368234
Liu Z, Yeung K (2008) The preconditioning and stress relaxation of skin tissue. J Biomed Pharm Eng 2:22–28
Simmonds N, Miller P, Gemmel H (2010) A theoretical framework for the role of fascia in manual therapy. J Bodywork Mov Ther 16:83–93. doi:10.1016/j.jbmt.2010.08.001
Stecco A, Macchi V, Masiero S, Porzionato A, Tiengo C, Stecco C, Delmas V, De Caro R (2009) Pectoral and femoral fasciae: common aspects and regional specializations. Surg Radiol Anat 31:35–42. doi:10.1007/s00276-008-0395-5
Stecco C, Pavan PG, Porzionato A, Macchi V, Lancerotto L, Carniel EL, Natali A, De Caro R (2009) Mechanics of crural fascia: from anatomy to constitutive modeling. Surg Radiol Anat 31:523–529. doi:10.1007/s00276-009-0474-2
Stecco C, Porzionato A, Lancerotto L, Stecco A, Macchi V, Day JA, De Caro R (2008) Histological of the deep fascia of the limbs. J Bodywork Mov Ther 12:225–230. doi:10.1016/j.jbmt.2008.04.041
Sverdlik A, Lanir Y (2002) Time-dependent mechanical behavior of sheep digital tendons, including the effects of preconditioning. J Biomech Eng 124:78–84. doi:10.1115/1.1427699
Turnipseed WD, Hurschler C, Vanderby R (1995) The effects of elevated compartment pressure on tibial arteriovenous flow and relationship of mechanical and biomechanical characteristics of fascia to genesis of chronic anterior compartment syndrome. J Vasc Surg 21:810–817. doi:10.1016/S0741-5214(05)80012-6
Acknowledgments
The mechanical testing instrument adopted for the development of this work was funded by CARIPARO Foundation (Italy). This support is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stecco, C., Pavan, P., Pachera, P. et al. Investigation of the mechanical properties of the human crural fascia and their possible clinical implications. Surg Radiol Anat 36, 25–32 (2014). https://doi.org/10.1007/s00276-013-1152-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-013-1152-y