Abstract
Purpose
We report a case of type 1 proatlantal intersegmental artery (PIA) associated with multiple anomalies of the aortic arch, and discuss the possible embryonic mechanism and clinical importance of the multiple cerebrovascular variants in this patient.
Methods
A 65-year-old woman with dizziness underwent cerebral magnetic resonance (MR) imaging and head and neck MR angiography using a 3-tesla scanner and computed tomography (CT) angiography using a 64-slice multidetector CT scanner.
Results
MR and CT angiography demonstrated an aneurysm of the distal end of the azygos anterior cerebral arteries and hypoplasia of the proximal right vertebral artery (VA) with an anastomotic artery, between the right internal carotid artery (ICA) and distal right VA that passed through the foramen magnum, indicating a type 1 PIA. She also demonstrated an aberrant right subclavian artery (ARSA) with hypoplasia of the right VA, and the left VA arose directly from the aortic arch.
Conclusion
To our knowledge, this is the first report of a type 1 PIA associated with multiple vascular anomalies of the aortic arch, such as ARSA and origin of the left VA from the arch. In cases of persistent anastomoses between the carotid and vertebrobasilar arteries, such as PIAs, imaging examination should include the aortic arch to identify associated vascular variations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A proatlantal intersegmental artery (PIA) is a rare congenital vascular anomaly and one of four types of persistent fetal anastomosis between the carotid and vertebrobasilar arteries. From caudal to cranial, the types are PIA, hypoglossal artery, otic artery, and trigeminal artery [8, 15]. Associated vascular abnormalities and variations with PIA are reported [1, 2, 7, 14, 17, 18]. We present a rare case of type 1 PIA associated with cerebral aneurysm and aberrant right subclavian artery (ARSA) with hypoplasia of the right vertebral artery (VA) and with left VA arising from the aortic arch, and we discuss the possible embryonic mechanism and clinical importance of the multiple cerebrovascular variants in this case.
Case report
We evaluated a 65-year-old woman with a history of hypertension, hyperlipidemia, and Hashimoto’s disease for dizziness. Cerebral magnetic resonance (MR) imaging showed multiple small old cerebral infarcts, and MR angiography of the head and neck using non-contrast three-dimensional time-of-flight technique demonstrated an aneurysm of the distal end of the azygos anterior cerebral arteries and hypoplasia of the right VA with an anastomotic artery between the right internal carotid artery (ICA) and distal right VA that passed through the foramen magnum (Fig. 1) but did not enter any of the transverse foramina of the cervical vertebrae. We therefore diagnosed PIA of type 1. To evaluate the aneurysm for treatment, we performed additional CT angiography of the head and neck from the aortic arch to the intracranial region which revealed an aberrant right subclavian artery (ARSA) that had a retroesophageal course with hypoplasia of the right VA and with left VA arising directly from the aortic arch, between the left common carotid artery (CCA) and left subclavian artery (SA) (Fig. 2). The source images of CT angiography also showed diffuse enlargement of the thyroid gland, which we attributed to her history of Hashimoto’s disease (not shown). We followed the aneurysm closely without treatment.
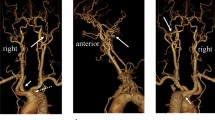
Magnetic resonance (MR) angiography demonstrating an anastomotic artery between the right internal carotid artery (ICA) and right vertebral artery (VA) (thick arrows). The proximal segment of the right VA is hypoplastic. Also note an aneurysm of the distal end of the azygos anterior cerebral arteries (thin arrow)
Computed tomographic (CT) angiography demonstrating an aneurysm of the distal end of the azygos anterior cerebral arteries (thin white arrow), an aberrant right subclavian artery (arrowhead) with hypoplasia of the right vertebral artery (VA), and the left VA arising directly from the aortic arch (thick black arrow)
Discussion
PIA is one of four types of persistent fetal anastomosis between the carotid and vertebrobasilar arteries. Lasjaunias and associates [8] initially classified two types that both arise from the carotid artery and enter the foramen magnum. Type 1 PIA corresponds to the first segmental artery, arising from the origin of the cervical ICA and running dorsally to the first cervical space, then passing in the cervicocervical space, and joining its opposite counterpart ventral to the spinal-medullary junction. Type 2 PIA arises laterally from the external carotid artery (ECA), remains more lateral in position than the type 1 PIA, and joins the course of the horizontal portion of the VA before entering the foramen magnum. Quijano and colleagues [12] reported that 38 % of PIAs they described were of type 1, 57 % were type 2, and 5 % arose from the CCA.
Associated vascular variations have been reported with PIA [2, 7, 14, 17, 18], with VA anomalies most common. Among their cases in which PIA persisted, Kolbinger’s group [7] found that the ipsilateral, contralateral, or both VAs were 46 % hypoplastic. It can be theorized that the persistence of carotid-basilar anastomoses, including PIAs, is related to a teleologic effort to preserve blood flow to the posterior fossa that has been compromised by hypoplastic or aplastic VAs [6]. Basekim and associates [2] also reported an aortic arch variation with PIA that involved a left brachiocephalic trunk of aortic origin that gave rise to the left CCA and SA. In our case, the ipsilateral VA was hypoplastic and originated from the ARSA. To the best of our knowledge, this is the first report of a type 1 PIA associated with vascular anomalies of the aortic arch, such as ARSA and left VA originating from the arch.
ARSA is an anomaly with reported incidence of 0.5–2 % [13]. Edwards [3] has hypothesized a double aortic arch system comprising an aortic arch and ductus arteriosus on each side, with right carotid artery and SA arising from the right arch and with left carotid artery and SA originating from the left arch. A normal arch system results from interruption of the dorsal segment of the right arch between the right SA and descending aorta with regression of the right ductus arteriosus. However, various anomalies arise when the arch system is interrupted at different locations. ARSA occurs as a result of interruption between the right carotid artery and right SA, and its associated anomalies include a common origin of the CCAs, replaced VA, coarctation of the aorta, and right-sided aortic arch [4].
The most common VA variant, with a reported incidence of 2.4–5.8 %, is the left VA originating directly from the aortic arch between the left CCA and left SA [9], and this variant is thought to represent the persistence of the sixth intersegmental artery. Embryologically, the left SA develops from the C7 intercostal artery, and the VA arises from the postcostal longitudinal anastomosis between the C1 and C7 intercostal arteries and the cervical intercostal obliteration zone [5, 10]. Therefore, we attributed the aortic branching pattern in our patient to the interruption of the arch system between the right carotid artery and right SA combined with persistence of the left sixth intersegmental artery.
A 59 % incidence of cerebrovascular abnormalities has been reported in patients with PIA, 10 % of whom had intracranial aneurysms [7]. Arteriovenous and Galen’s vein malformations have also been associated with PIA [1, 11, 18], suggesting that some changes in congenital hemodynamic factors caused by a PIA might affect the pathogenesis of cerebrovascular abnormalities. In our case, however, we discovered the aneurysm only incidentally by its different location from the PIA.
Patients with PIAs and anomalies of the aortic arch are generally asymptomatic, but ARSA may cause dysphasia as a result of extrinsic compression of the esophagus caused by the vessel’s retroesophageal course [16]. As in our case, most PIAs are discovered incidentally, but it remains important to recognize these associated vascular anomalies, particularly before undertaking endovascular and surgical treatment of the brain, neck, and chest.
Conclusion
We present the first report of type 1 PIA associated with multiple vascular anomalies of the aortic arch, including ARSA and left VA originating from the aortic arch. Imaging examination should include the aortic arch to find other associated vascular variations and abnormalities in cases of persistent anastomoses, between the carotid and vertebrobasilar arteries like PIAs.
References
Arráez-Aybar LA, Navia-Álvarez P, Méndez-Cendón JC (2011) A case of a type II proatlantal artery with arteriovenous malformation. Surg Radiol Anat 33:85–89
Basekim CC, Silit E, Mutlu H, Pekkafali MZ, Ozturk E, Kizilkaya E (2004) Type I proatlantal artery with bilateral absence of the external carotid arteries. AJNR Am J Neuroradiol 25:1619–1621
Edwards JE (1948) Anomalies of the derivatives of the aortic arch system. Med Clin North Am 32:925–949
Epstein DA, Debord JR (2002) Abnormalities associated with aberrant right subclavian arteries-a case report. Vasc Endovascular Surg 36:297–303
Goray VB, Joshi AR, Garg A, Merchant S, Yadav B, Maheshwari P (2005) Aortic arch variation: a unique case with anomalous origin of both vertebral arteries as additional branches of the aortic arch distal to left subclavian artery. AJNR Am J Neuroradiol 26:93–95
Gumus T, Onal B, Ilgit ET (2004) Bilateral persistence of Type 1 proatlantal arteries: report of a case and review of the literature. AJNR Am J Neuroradiol 25:1622–1624
Kolbinger R, Heindel W, Pawlik G, Erasmi-Körber H (1993) Right proatlantal artery type 1, right internal carotid occlusion, and left internal carotid stenosis: case report and review of the literature. J Neurol Sci 117:232–239
Lasjaunias P, Berenstein A, ter Brugge KG (2001) Clinical vascular anatomy and variations, 2nd edn. In: Surgical neuroangiography, vol 1. Springer, Berlin
Lemke AJ, Benndorf G, Liebig T, Felix R (1999) Anomalous origin of the right vertebral artery: review of the literature and case report of right vertebral artery origin distal to the left subclavian artery. AJNR Am J Neuroradiol 20:1318–1321
Newton TH, Mani RL (1974) The vertebral artery. In: Newton TH, Potts DG (eds) Radiology of skull and brain. Mosby, St. Louis, pp 1659–1672
Purkayastha S, Gupta AK, Varma R, Kapilamoorthy TR (2005) Proatlantal intersegmental arteries of external carotid artery origin associated with Galen’s vein malformation. AJNR Am J Neuroradiol 26:2378–2383
Quijano J, Whipple SJ, Winkler MA, Kostanian V (2006) Proatlantal persistent segmental artery. http://rad.usuhs.mil/medpix/master.php3?mode=single&recnum=7175&table=&srchstr=&search=
Stewart JR, Kincaid OW, Edwards JE (1964) An atlas of vascular rings and related malformations of the aortic arch system. Charles C. Thomas, Springfield
Tanaka Y, Hara H, Momose G, Kobayashi S, Kobayashi S, Sugita K (1983) Proatlantal intersegmental artery and trigeminal artery associated with an aneurysm. Case report. J Neurosurg 59:520–523
Tubbs RS, Shoja MM, Salter EG, Oakes WJ (2007) Cadaveric findings of persistent fetal trigeminal arteries. Clin Anat 20:367–370
Türkvatan A, Büyükbayraktar FG, Ölçer T, Cumhur T (2009) Multidetector computed tomographic angiography of aberrant subclavian arteries. Vasc Med 14:5–11
Uchino A, Saito N, Inoue K (2011) Type 2 proatlantal intersegmental artery associated with persistent trigeminal artery diagnosed by MR angiography. Surg Radiol Anat Jun 19 (Epub ahead of print)
Vasović L, Mojsilović M, Anđelković Z et al (2009) Proatlantal intersegmental artery: a review of normal and pathological features. Childs Nerv Syst 25:411–421
Acknowledgments
We thank Rosalyn Uhrig, M.A., for editorial assistance in the preparation of this manuscript.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saito, N., Uchino, A. & Ishihara, S. Complex anomalies of type 1 proatlantal intersegmental artery and aortic arch variations. Surg Radiol Anat 35, 177–180 (2013). https://doi.org/10.1007/s00276-012-1017-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-012-1017-9