Abstract
Purpose
We present a case of an aberrant right subclavian artery (ARSA) with extremely rare vascular anomalies.
Case report
A 69-year-old woman was suspected to have right internal carotid artery (ICA) stenosis. Computed tomography angiography demonstrated an ARSA and hypoplasia of the right ICA. The proximal segment of the right vertebral artery (VA) was aplasia, and a right type 1 proatlantal artery (PA) arose from the right common carotid artery. Cerebral angiography demonstrated segmental dysplasia of the right ICA. The ascending intrapetrous segment and the ascending foramen lacerum-horizontal intracavernous segment of the right ICA demonstrated hypoplasia. The collateral pathways promoted reconstitution of each of the distal segments. Left internal carotid angiography demonstrated anterior communicating artery aneurysm and sufficient cross flow to the contralateral middle cerebral artery via the AcomA.
Discussion
A type 1 PA with an ARSA may result in the regression of the right dorsal aorta with persistence of the first cervical intersegmental artery. Although there are few findings of a relationship between an ARSA and intracranial artery anomalies, a developmental error of the right dorsal aorta may cause such complex vascular anomalies.
Conclusion
Knowledge of anatomical variations in patients with ARSA is useful when performing angiography or endovascular therapy, as well as during clinical follow-up.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An aberrant right subclavian artery (ARSA) is a congenital anomaly of the aortic arch with a reported incidence of 0.5%–2.2% [16]. An ARSA often coexists with other vascular anomalies, such as an anomalous origin of the vertebral artery (VA) or common carotid trunk [1, 6, 15]. However, few studies have reported an ARSA with carotid-basilar anastomosis or intracranial artery anomalies. Here, we report a rare case of an ARSA with a right type 1 proatlantal artery (PA) and segmental dysplasia of the right internal carotid artery (ICA).
Case report
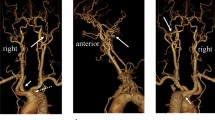
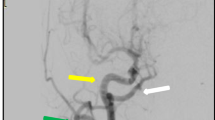
A 69-year-old woman presented with dizziness. After hospitalization, magnetic resonance angiography was performed, which revealed right ICA stenosis. Computed tomography angiography and cerebral angiography were performed, and an ARSA was confirmed. The right carotid canal exhibited hypoplasia (Fig. 1). The right VA arose from the right common carotid artery (CCA), did not enter the transverse foramen of the cervical vertebra, and ran directly between the atlanto-occipital space, indicating a type 1 PA. The right occipital artery (OA) arose from the cervical ICA. The left VA arose from the left subclavian artery and was normal in size. The ascending intrapetrous segment of the right ICA demonstrated hypoplasia. There was a collateral pathway from the ipsilateral VA to the caroticotympanic artery. Additionally, the ascending foramen lacerum-horizontal intracavernous segment of the right ICA exhibited hypoplasia. There was a collateral pathway from the external carotid artery (ECA) to the inferolateral trunk. An anterior communicating artery (AcomA) aneurysm and sufficient cross flow to the right middle cerebral artery territory from the left ICA via the AcomA were observed (Fig. 2).
a Lateral view of the right common carotid angiography. The type 1 PA (blue arrow) originated from the right CCA. The blue arrowhead indicates the orifice of the type 1 PA. The ascending intrapetrous segment of the right ICA exhibited hypoplasia (red arrow). The caroticotympanic artery from the ipsilateral VA flowed out just distal to the hypoplastic segment (white arrowhead). The right OA (white arrow) arose from the cervical ICA. b Lateral view of the right external carotid angiography in the early arterial phase and c arterial phase. The ascending foramen lacerum and horizontal intracavernous segments of the right ICA exhibited hypoplasia. There was a collateral pathway from the inferolateral trunk. The horizontal intrapetrous segment was normal. d Antero-posterior view of the right external carotid angiography. e Three-dimensional digital subtraction angiography oblique lateral view of the right common carotid angiography. f Antero-posterior view of the left common carotid angiography demonstrated an AcomA aneurysm and sufficient cross flow to the right middle cerebral artery territory from the left ICA via the AcomA
Discussion
The primitive ICA forms from the extended dorsal aorta and the third aortic arch. The bilateral dorsal third aortic arches regress until the embryo is 18 mm, and the ECA and ICA connect to the CCA. Until the embryo is 40 mm, the distal portion of the right dorsal aorta is regressed, but its proximal portion remains as the innominate artery (Fig. 3a) [5]. An ARSA forms from the persistence of the right distal dorsal aorta and regression of the right fourth aortic arch and right dorsal aorta proximal to the seventh cervical intersegmental artery (CIA) (Fig. 3b). As such, an ARSA can occur during embryonic development.
Schematic illustrations of aortic arch development (modified from Ref. [5]). a Normal development. b ARSA results from regression of the distal dorsal aorta from the right fourth aortic arch with persistence of the right seventh CIA. c ARSA with a right type 1 PA originated from the CCA results from regression of the right dorsal aorta with persistence of a first CIA. Different from simple ARSA, the right fourth aortic arch remains and connects to the right CIA. d Segmental concept of the ICA by Lasjaunias et al. [7]. Each segment lies between the embryonic arteries or their remnants. ICA segments are numbered 1–7, as follows: 1 = cervical; 2 = ascending intrapetrous; 3 = horizontal intrapetrous; 4 = ascending foramen lacerum; 5 = horizontal intracavernous; 6 = clinoid; and 7 = termination. In the present case, the ascending intrapetrous and ascending-horizontal intrapetrous segments exhibited hypoplasia (filled in red). e Schema of the ICA in the later stage of embryonic development. The corresponding segments were hypoplasia, and all were part of the dorsal aorta (filled in red). ARSA aberrant right subclavian artery, CCA common carotid artery, CIA cervical intersegmental artery, DA dorsal aorta, ECA external carotid artery, IA innominate artery, ICA internal carotid artery, ILT inferolateral trunk, PMA primitive mandibular artery, POA primitive ophthalmic artery, PA proatlantal artery, SCA subclavian artery, TCT thyrocervical trunk, VA vertebral artery, VeA ventral aorta, I first aortic arch, II second aortic arch, III third aortic arch, IV fourth aortic arch (colour figure online)
An ARSA often coexists with cervical artery anomalies, such as anomalies of the common carotid trunk and an abnormal right VA origin, such as the right CCA or ICA [1, 2, 6, 13, 15]. Tsai et al. [15] reported that the right VA arises from the right CCA in 13.7% of cases of ARSA. Commonly, this results from regression of the right mid-dorsal aorta with persistence of a third, fourth, or fifth CIA [5]. In such cases, the remaining right seventh CIA connects to the left distal dorsal aorta, leading to ARSA formation. Although this anomaly looks like an ARSA subtype, the right fourth aortic arch remains and connects to the right CIA [2, 5]. Thus, an ARSA with an anomalous right VA origin is different from an ARSA that develops embryologically.
In the present case, the right VA indicated a right type 1 PA which is one of the five carotid-vertebrobasilar anastomoses (primitive persistent trigeminal, otic, hypoglossal, type 1 PA, and type 2 PA). Until the embryo is 4 mm, the paired longitudinal neural arteries are supplied by the carotid arteries via fetal carotid-vertebrobasilar anastomoses. Until the embryo is 5–6 mm, the anastomosis that later becomes the posterior communicating artery develops, and the fetal carotid-vertebrobasilar anastomoses subsequently regress and are obliterated [11]. The remnants of these fetal carotid-vertebrobasilar anastomoses are rarely confirmed in adults. A type 1 PA corresponds to the first CIA, arises from the carotid arteries at the second through fourth levels of the cervical vertebrae, joins the VA, and runs between atlanto-occipital space. A type 1 PA often arises from the ICA, but it may also arises from the CCA [11]. It has been reported that if the first or second CIA persists, the VA abnormally arises from the ICA or ECA. Additionally, if the third through sixth CIAs persists, the VA abnormally arises from the CCA or aortic arch [2]. The VA normally forms from the longitudinal anastomosis of the CIAs, and enters the transverse foramen of the sixth cervical vertebra. The entry of the VA into the transverse foramen is dependent on the correspondent intersegmental artery [12]. The VA, which results from the persistence of the lower CIA, is considered to enter the transverse foramina of the third to sixth cervical vertebrae. Thus, although the right VA in the present case arose from the right CCA, it was not the remnant of the lower CIA but instead a type 1 PA, which resulted from the regression of the right dorsal aorta and persistence of the first CIA and right fourth aortic arch (Fig. 3c). In the present case, the right OA arose from the right ICA. Lasjaunias et al. hypothesized that the proximal segment of the OA is the remnant of the proatlantal intersegmental artery; thus, it is considered that the OA arising from the ICA is the persistent proatlantal intersegmental artery [9]. Because the OA originated from the proximal cervical ICA, the orifice of the type 1 PA might have lowered to the CCA. There are few reports of a PA with an ARSA [14, 16]. Because the development of a PA is completed before that of an ARSA, so the anomalous origin of the right VA including a right PA associated with an ARSA (which is caused by the regression of the right dorsal aorta) may be embryologically different from an isolated PA (which is unrelated to an ARSA).
Lasjaunias et al. [7] suggested that the ICA is made up of seven segments, each of which lies between embryonic arteries or their remnants. In case of segmental agenesis of the ICA, the collateral pathway immediately distal to the missing segment promotes reconstitution [7]. In the present case, we diagnosed hypoplasia at the ascending intrapetrous segment and the ascending foramen lacerum-horizontal intracavernous segment because of the relationship between the collateral pathways and the contrast effect of angiography. The ascending intrapetrous segment develops from the dorsal aorta between the second and third aortic arches, while the ascending foramen lacerum-horizontal intracavernous segment develops from the dorsal aorta between the first aortic arch and the inferolateral trunk (Fig. 3d, e).
There are no reports of the embryological relationship between an ARSA and ICA dysplasia. An ARSA with a right VA originating from the CCA results from partial regression of the right distal dorsal aorta, as mentioned above. Similarly, segmental dysplasia of the ICA results from regression of the intracranial distal dorsal aorta. Thus, it is considered that an ARSA with an anomalous origin of the right VA and segmental dysplasia of the right ICA might be explained centrally as multiple segmental regression of the right dorsal aorta. The fact that all vascular anomalies in the present case occurred on the right side supports this hypothesis. Dysplasia of a single segment of the ICA has been reported previously, but there are few reports of separate segmental aplasia or hypoplasia of more than two segments of the ICA. In general, developmental errors occur in a step-by-step fashion. For example, ICA aplasia results from agenesis of one ICA segment first and of adjacent segments thereafter, and progressive aplasia of adjacent segments promotes ICA aplasia [8]. Although hypoplasia may incidentally occur in separate segments, it cannot be explained as a focal developmental error considering the combination of rare vascular anomalies of the present case. Research has shown that a PA frequently coexists with other cerebrovascular abnormalities [14]. Congenital hemodynamic factors such as decreasing antegrade flow of the ICA caused by a PA might affect the occurrence of the ICA dysplasia. Moreover, considering the relationship with intracranial and extracranial vascular anomalies, multi-segmental regression or a systemic developmental error of the right dorsal aorta might resulted in an ARSA with a right type 1 PA and dysgenesis of the right ICA.
PHACE syndrome causes multiple intracranial arterial anomalies and other characteristics such as posterior fossa anomalies, hemangioma, coarctation of the aorta, and eye anomalies [4]. Because our patient had no remarkable medical history and had developed normally, so her multiple arterial anomalies were unrelated to PHACE syndrome.
The circle of Willis is completely developed when the AcomA is formed in the 24 mm embryo. Dysplasia of the ICA may occur in the 20–24 mm embryo; thus, the collateral pathway via the AcomA tends to be well developed [10]. Segmental dysplasia of the ICA may cause a cerebral aneurysm, which most often occurs at the AcomA complex due to hemodynamic stress [3]. Conversely, cerebral hypoperfusion in the ipsilateral hemisphere of ICA dysgenesis may occur [3]. There is a possibility that associated conditions result in subarachnoid hemorrhage or ischemic stroke, so clinical and radiological follow-up is needed.
In conclusion, an ARSA often coexists with an anomalous VA origin, but it rarely coexists with a right type 1 PA and dysplasia of the right ICA. There may be an embryological relationship with the right dorsal aorta. Knowledge of anatomical variations in patients with an ARSA is useful when performing angiography or endovascular therapy, as well as during clinical follow-up.
References
Buffoli B, Verzeletti V, Hirtler L, Rezzani R, Rodella LF (2021) Retroeshophageal right subclavian artery associated with a bicarotid trunk and an ectopic origin of vertebral arteries. Surg Radiol Anat 43:1491–1495. https://doi.org/10.1007/s00276-021-02746-1
Chen C, Wang L, Wong Y (1998) Abnormal origin of the vertebral artery from the common carotid artery. Am J Neuroradiol 19:1414–1416
Gailloud P, Clatterbuck RE, Fasel JH, Tamargo RJ, Murphy KJ (2004) Segmental agenesis of the internal carotid artery distal to the posterior communicating artery leading to the definition of a new embryologic segment. Am J Neuroradiol 25:1189–1193
Garzon MC, Epstein LG, Heyer GL et al (2016) PHACE syndrome: consensus-derived diagnosis and care recommendations. J Pediatr 178:24-33.e2. https://doi.org/10.1016/j.jpeds.2016.07.054
Haughton VM, Rosenbaum AE (1974) The normal and anomalous aortic arch and brachiocephalic arteries. In: Newton TH, Potts DG (eds) Radiology of the skull and brain, vol 2. Book 2. Arteries. Mosby, St Louis, pp 1145–1163
Kumar S, Kumar P (2014) Truncus bicaroticus with aberrant right subclavian artery and origin of right vertebral from right common carotid artery. Surg Radiol Anat 36:829–831. https://doi.org/10.1007/s00276-013-1232-z
Lasjaunias P, Berenstein A (1987) Arterial Anomaly: Introduction. In: Lasjaunias P, Berenstein A (eds) Surgical neuroangiography, volume 1, Functional anatomy of craniofacial arteries, 1st edn. Springer-Verlag, Berlin, pp 1–32
Lasjaunias P, Santoyo-Vazquez A (1984) Segmental agenesis of the internal carotid artery: angiographic aspects with embryological discussion. Anat Clin 6:133–141. https://doi.org/10.1007/BF01773165
Lasjaunias P, Théron J, Moret J (1978) The occipital artery. Anatomy-normal arteriographic aspects-embryological significance. Neuroradiology 15:31–37. https://doi.org/10.1007/BF00327443
Lee JH, Oh CW, Lee SH, Han DH (2003) Aplasia of the internal carotid artery. Acta Neurochir 145:117–125. https://doi.org/10.1007/s00701-002-1046-y
Luh GY, Dean BL, Tomsick TA, Wallace RC (1999) The persistent fetal carotid-vertebrobasilar anastomoses. Am J Roentgenol 172:1427–1432
Newton TH, Mani RL (1974) The vertebral artery. In: Newton TH, Potts DG (eds) Radiology of the skull and brain, vol 2. Book 2. Arteries. Mosby, St Louis, pp 1659–1709
Ota T, Fujitani S, Fujimoto S, Saito H, Ueda M (2020) A rare anomalous origin of the right vertebral artery from the internal carotid artery with an aberrant right subclavian artery. World Neurosurg 139:250–252. https://doi.org/10.1016/j.wneu.2020.04.080
Saito N, Uchino A, Ishihara S (2013) Complex anomalies of type 1 proatlantal intersegmental artery and aortic arch variations. Surg Radiol Anat 35:177–180. https://doi.org/10.1007/s00276-012-1017-9
Tsai IC, Tzeng WS, Lee T et al (2007) Vertebral and carotid artery anomalies in patients with aberrant right subclavian arteries. Pediatr Radiol 37:1007–1012. https://doi.org/10.1007/s00247-007-0574-2
Uchino A, Tokushige K (2022) Type 2 left proatlantal artery with normal left vertebral artery and association with an aberrant right subclavian artery and a bi-carotid trunk. Surg Radiol Anat. https://doi.org/10.1007/s00276-022-02896-w
Funding
No funding.
Author information
Authors and Affiliations
Contributions
KI: project development, data collection and analysis, literature research and manuscript writing. HE: literature research, manuscript editing. KS: data analysis and literature research. RN: data collection and analysis. KO: data collection and management. HN: project development and total management.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The ethics committee responsible (the Ethics Committee of Nakamura Memorial South Hospital) issued a waiver in written form for case reports.
Informed consent
Informed consent was obtained from this patient.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ishikawa, K., Endo, H., Shindo, K. et al. Aberrant right subclavian artery with right type 1 proatlantal artery and segmental dysplasia of the right internal carotid artery: a case report. Surg Radiol Anat 44, 709–713 (2022). https://doi.org/10.1007/s00276-022-02950-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-022-02950-7