Abstract
Purpose
The endoscopic transnasal, transsphenoidal approach is considered by many a valid option to reach the sellar region and, in selected cases, to decompress the optic nerve. However, few data are available in literature about the real effectiveness of the procedure and the extent of nerve decompression needed to obtain a clinical result. The aim of this anatomical study was to describe the most important landmarks of the endoscopic transsphenoidal approach to the optic nerve.
Methods
Six silicone-injected cadaver heads were dissected via the endoscopic transnasal approach, performing a bilateral optic nerve decompression. The lateral optocarotid recess (OCR) and optic canal were identified in each case. Moreover, the relationship between the ophthalmic artery at its origin and the optic nerve was examined.
Results
Twelve decompressions of the optic nerve were performed, obtaining the following measurements: intercarotid distance 12 mm ± 1.5, median length of OCR 5 mm ± 1 and average length of optic nerve decompression 15 mm ± 2. The ophthalmic artery was observed emerging from the internal carotid artery (ICA) medially in six cases, ventrally in four cases and laterally in two cases.
Conclusion
A wide optic nerve decompression may be obtained with transsphenoidal approach. However, the risk of ophthalmic artery injury seems to be more relevant than with supratentorial approaches, due to the intimate relationship between artery and nerve on its inferior surface. Knowledge of anatomical landmarks, such as lateral OCR and the position of the ophthalmic artery, is useful to prevent this injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The sellar region is a complex part of the skull base because of its multiple anatomical structures: among all, the optic nerves. For this reason, the region could be defined both from an anatomical and a functional point of view [20, 21]. Many surgical approaches have been described to reach this region [7, 10, 16, 22, 23, 25].
One classification distinguishes between intracranial and transsphenoidal approaches, the last ones being considered less invasive than the supratentorial ones [8].
More recently, the transsphenoidal endoscopic technique has been considered by some authors as a new and more valid alternative than the “classical” microsurgical one [3, 8, 12]. This surgical approach has been chosen, in selected cases, to decompress the optic nerve [5, 13, 14]. Craniofacial trauma, medical refractory pseudotumor and thyrotoxic orbitopathy are examples of such cases [14, 18, 19, 24], even if the usefulness of decompressing the II cranial nerve in its intracanalicular part is still debated [19].
In this paper, we have performed an anatomical description of the course of the optic nerve during a transsphenoidal approach performed to decompress the nerve itself.
The main objectives of the study are to describe the most significant structures and landmarks encountered during this procedure, the aspects of anatomy that are relevant to surgery and the extension of nerve decompression that may be obtained with this approach.
Measurements of dimensions and reciprocal relationships of the optic nerve with the carotid and ophthalmic artery were reported, underlying the importance of their anatomical variability. Moreover, we compared the transsphenoidal approach with the intracranial one, discussing the pros and cons of the two options.
Materials and methods
Six human cadaveric fresh heads were used. On these heads, only the arterial system was injected with red colored silicone rubber (Dow Corning Corp., Midland, MI, USA). All dissections were performed at the Institute of Anatomy of the University of Vienna.
Rigid endoscopes that were 4 mm in diameter, 18 cm in length, with 0° and 45° lenses (Karl Stortz GmbH & Co, Tuttlingen, Germany) were used. The endoscope was connected to a light source via a fiberoptic cable and to a camera. The video camera was connected to a 21-inch monitor. To guarantee a suitable file of anatomical images, a digital video recorder system (DVCam) was used.
A standard endoscopic endonasal approach to the sphenoid sinus was performed in each case, using a 0° lens endoscope in the initial phase. Cadavers with great or well-pneumatized sphenoid sinus make this approach easier. Bilateral optic nerve decompression was performed on each specimen. The endoscope was initially introduced through the right nostril, close to the floor of the nasal cavity, leading to the identification of the nasal septum, and the inferior and the middle turbinate. With a gentle lateral dislocation of the head of the middle turbinate, an adequate surgical corridor toward the posterior nasal cavity was obtained. Advancing the endoscope, the choana, which represents the inferior landmark of the approach, was visualized. As the choana was reached, the endoscope was angled rostrally, along the roof of the choana and the sphenoethmoid recess.
The sphenoid ostium was usually identified approximately 1.5 cm above the roof of the choana. Sometimes, the ostium was covered by the superior turbinate and its identification was difficult: in these cases, it was necessary to lateralize the superior turbinate or to remove it, to gain access to the sphenoidal sinus. After entering the sphenoid sinus through the ostium, the anterior wall of the sinus was removed. The sphenoid rostrum was separated from the nasal septum and a standard anterior sphenoidotomy was performed. The extent of the anterior sphenoidotomy was comparable to that usually performed during pituitary surgery. After the anterior sphenoidotomy was completed, septa inside the sphenoid sinus were eventually removed with Kerrison rongeurs. After accurate removal of the sphenoid septa, full visualization of posterior and lateral walls of the sinus was obtained.
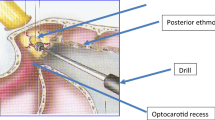
The bony anatomical landmarks of the sphenoid sinus were then identified: the optic nerve protuberances, the carotid protuberance and the lateral optocarotid recess (OCR) at about the 10 o’clock and the 2 o’clock points laterally to the sellar floor.
The optic nerve protuberance is the bulge made by the optic canal on the sinus cavity side of the lateral sphenoid sinus wall.
The carotid protuberance belongs to the lateral wall of the body of the sphenoid bone. The anterior bend (C3 segment) of the internal carotid artery (ICA) courses along to this bone and forms the osseous convolutions on the inner surface of the sphenoid sinus.
The lateral optocarotid recess represents the pneumatization of the optic strut of the anterior clinoid process. It is located between the osseous prominences of the internal carotid artery (ICA) below and of the optic nerve above.
Optic nerve decompression was then performed from the chiasm to the distal wall of the orbit (the decompression was stopped with the identification of the lamina papyracea and the Zinn annulus). The decompression was obtained with the use of the Hardy dissector and with a high speed microdrill when the bone was too thick.
For every specimen it was possible to obtain a wide bilateral decompression of the optic nerve from the region of the chiasm to the lateral wall of the orbit.
The measurements obtained were:
-
the distance between carotid arteries and optic nerves protuberances,
-
the extent of optic nerve decompression.
The second part of the study was focused on the anatomical relationship between the ophthalmic artery at its origin, the carotid artery and the optic nerve.
Results
Decompression of the optic nerve was achieved in all the six human cadaveric fresh heads bilaterally.
The surgical approach was the “pure” endoscopic transnasal approach described to reach the pituitary gland and sellar region. As documented, the key anatomical landmarks were recognized and highlighted during every step of the approach.
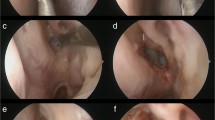
In the first phase of the procedure (nasal one), we focused on the choana/aditus, the rhinopharyngeal region, the middle turbinate and the sphenoidal ostium (1.5 cm above the choana) (Fig. 1a).
a Endonasal step: visualization of sphenoid ostium (SO). b Sphenoid step: removal of septum, carotid and optic identification, and measurement of intercarotid distance. c Visualization of the optic nerve protuberance (OP), the carotid artery protuberance (CP) and the optic-carotid recess (OCR). d Measurement of distance between the carotid artery and the optic nerve
In the second phase (sphenoidal one), attention was shifted to the sphenoidal septum, carotid artery protuberance (CP), optic nerve protuberance (OP) and the lateral OCR.
The following measurements were obtained: intercarotid distance (the median distance between the two CPs) of 12 mm ± 1.5; median length of lateral OCR (which corresponds to the distance between the parasellar carotid artery and the optic nerve) of 5 mm ± 1 (Fig. 1b, c).
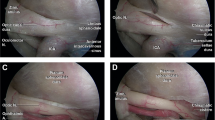
In the third phase (optic nerve one), the bony wall surrounding the nerve was removed (Fig. 2a). The average length of optic nerve after decompression was 15 mm ± 2 (Fig. 2b). This measurement was greater than the length of optic canal classically reported in previous anatomical studies.
The explanation of this difference was the extension of the decompression, which was conducted from the orbit to the chiasm, so that the part of nerve exposed was not only the intracanalicular one but also the intracranial and orbit ones. The unfolding of “lamina papyracea” and the Zinn’s annulus confirmed the correct exposure of the orbit (Fig. 2c, d).
The exposition of the ophthalmic artery, to demonstrate its relationship with the optic nerve, was another aim of the study (Fig. 3a). In the specimens, the artery emerged from the internal carotid artery inferomedial to the nerve in 6/12 decompressions (50%), inferior in 4/12 (30%) and inferolateral in 2/12 (20%). The entry of the ophthalmic artery into the optic canal followed the same topography with the same percentages. In the same specimen, no difference was remarked between the two sides.
To verify the measurements, all the bony parts surrounding the arteries and nerves were removed. In this way, the communicating artery–anterior cerebral arteries complex, the ophthalmic artery, and the pituitary gland and its stalk were exposed (Fig. 3b).
Discussion
The great number of vascular and nervous structures inside the sellar region makes this portion of the skull base one of the most complex in the human body. Because of this complexity, it represents a real challenge to neurosurgeons.
Many different approaches, both transcranial and transsphenoidal, have been described to reach this region. Among the transsphenoidal approaches, the endoscopic one has gained popularity in the last few years. Its low invasiveness and the possibility of an excellent visualization are the main reasons for its popularity. This approach has been proposed for treating lesions of the sellar and parasellar regions such as pituitary adenomas, meningiomas and craniopharyngiomas. Crucial in this approach is the intraoperative use of neuroradiological examinations, such as fluoroscopy and/or neuronavigation with CT/MR scan, to identify the anatomical landmarks.
Decompression of the optic nerve represents one of the surgical indications for this approach. According to classical anatomical descriptions [20], the second cranial nerve course could be divided into four parts, starting from the optic papilla. The first one is the intraocular one and is made of the amyelinic fibers arising form the optic papilla. Leaving the ocular bulb, these fibers acquire myelin and the three meningeal sheaths. The second one is the orbital portion: its length is about 30 mm. The third one is the intracanalicular one (8 mm) from the orbit to the optic foramen. The fourth is the intracranial part. It ends at the chiasm and is about 10 mm long. The properly defined optic canal is represented by the bony part of the optic course.
Optic nerve decompression is a surgical technique used in selected pathological conditions such as pseudotumor cerebri and craniofacial trauma [18, 19]. The aim of this procedure was to remove the bony wall of the optic canal to relieve the edematous nerve. This surgical strategy is a well-known therapeutical concept and is applied in different pathological conditions in neurosurgery. However, decompression of the optic nerve remains a procedure that is not yet completely accepted. The difficulty in evaluating the real effectiveness of this procedure compared to its elevated risks is among the reasons for the controversy. Moreover, surgical series are always inadequate in numbers and incomplete in clinical, radiological and follow-up evaluation. The first and classical approach described in literature for decompression of the optic nerve is the transcranial one (pterional with both an extradural and intradural recognition and decompression of the nerve). Over the last few years, the endoscopic transsphenoidal approach for sellar and parasellar lesions has gained popularity [2, 4, 9].
In the present study, we have illustrated decompression of the optic nerve by an endoscopic transsphenoidal approach, which in the first part (nasal one) is not different from the “pure” endoscopic approach to the pituitary gland.
Landmarks that identify the most relevant anatomical structures were highlighted to avoid “dangerous” situations and to define the optic course. We were able to identify the OP and the OCR in all specimens. As in previous studies [17], we found the lateral OCR to be the most reliable landmark that allowed identification of the optic nerve and its direction, the internal carotid and ophthalmic artery.
Our measurements (intercarotid distance, length of lateral OCR and length of optic nerve) showed significant decompression of the optic nerve, starting from the optic recess to the medial part of the orbit and to the chiasm. The average length of the decompressed optic nerve measured 15 mm ± 2. This measure represents the exposure of the nerve both in the intracanalicular part and in the intracranial one.
Knowledge of these landmarks and measurements are essential to avoid fatal mistakes and to obtain a proper space of work. Use of a micro-Doppler probe to discover the ICA, however, must be considered when the intercarotid distance is small (“kissing” of carotid arteries), or when there is uncertainty about the localization of the carotid protuberances, as in reinterventions.
We also demonstrated a great variability in the ophthalmic artery at its origin, with different sectors of entrance in the optic canal by the artery. In the majority of specimens (50%), the artery was found to be inferomedial to the nerve at the intracranial opening of the optic canal, whereas it was inferior to the nerve in the optic canal in the majority of cases. Based on this observation, it is safer to open the optic sheath in its medial part while decompressing the nerve in the optic canal. These anatomical findings are very similar to those reported in previous studies [11, 15, 17].
In 30% of our specimens, the artery originates from the internal carotid artery directly inferior to the nerve at the intracranial opening of the canal. This finding has also been reported in a previous study by Li et al. in 19% of cases [17]. In this study, the authors were also able to demonstrate a bony canal (the ophthalmic canal), located within the inferior optic canal wall. From a surgical point of view, one should keep in mind that the ophthalmic artery can be injured while working near the intracranial opening of the canal, when its inferior wall is drilled. Knowledge of the position and anatomical relationships of the artery with the optic nerve is crucial during the exposed surgical procedure. The anatomical variability of these structures underlines the importance of detailed neuroradiological studies (e.g., an angio-CT) before the procedure.
In our opinion, the main advantages of this approach are the extension of the decompression obtained and its relative safety. Another important reason in favor of the transnasal approach is the surgical anatomy of the pterional approach. This approach allows for direct exposure of the anterior clinoid process, which forms the lateral margin of the optic canal, and of the dural folds that are attached to this process. The falciform ligament is a dural fold that extends from the anterior clinoid process across the top of the optic nerve, just proximal to the optic canal, to the tuberculum sellae. This fold continues posteriorly to form the anterior petroclinoid fold, which is the free edge of the tentorium.
The falciform ligament seems to be in the ideal position to guillotine the optic nerve [20, 21]. The necessity to cut the ligament during the cranial strategy due to its position is avoided in the proposed endoscopic technique. Moreover, the anterior clinoid process is spared using this technique, and extension of decompression obtained in our specimens (involving at least two different sectors of the anatomical course) resulted to be wider than that achievable by the transcranial approach [1, 6].
Risk of cerebrospinal fluid (CSF) leak may represent a problem after optic nerve decompression, especially if completed by dural sheath fenestration. This risk is related to the anatomy of the prechiasmatic cistern, which sometimes is well developed and extends inferiorly toward the diaphragm, and laterally to include the intracranial part of the optic nerves. Opening of this cistern during nerve decompression may lead to postoperative leaks. Different strategies have been described in the literature to solve CSF leaks [2, 4].
Low invasiveness and wider exploration of the operative field are the cardinal points of strength of the endoscopic technique.
Conclusion
Transsphenoidal approach may be considered as an alternative way to perform optic nerve decompression.
Although the data reported here should be considered carefully, this study being an anatomical and not a surgical one, we conclude that decompression of the II cranial nerve obtained during trassphenoidal approach is satisfactory.
The main advantages of this technique are the low invasiveness, avoidance of cutting the falciform ligament and the possibility of a wide exploration of the operative field.
Exact knowledge of the variability of the origin of the ophthalmic artery and its anatomical relationship with the internal carotid artery and optic nerve are crucial for this approach.
References
Avci E, Bademci G, Ozturk A (2005) Microsurgical landmarks for safe removal of anterior clinoid process. Min Invasive Neurosurg 48(5):268–272
Cappabianca P, Cavallo LM, Esposito F, De Divitiis O, Messina A, De Divitiis E (2008) Extended endoscopic endonasal approach to the midline skull base: the evolving role of transsphenoidal surgery. Adv Tech Stand Neurosurg 33:151–199
Catapano D, Sloffer CA, Frank G, Pasquini E, D’Angelo VA, Lanzino G (2006) Comparison between the microscope and endoscope in the direct endonasal extended transsphenoidal approach: anatomical study. J Neurosurg 104(3):419–425
Cavallo LM, De Divitiis O, Aydin S, Messina A, Esposito F, Iaconetta G, Talat K, Cappabianca P, Tschabitscher M (2008) Extended endoscopic endonasal transsphenoidal approach to the suprasellar area: anatomic considerations, part 1. Neurosurgery 62(6 Suppl 3):1202–1212
Chen C, Selva D, Floreani S, Wormald PJ (2006) Endoscopic optic nerve decompression for traumatic optic neuropathy: an alternative. Otolaryngol Head Neck Surg 135(1):155–157
Collignon F, Link M (2005) Paraclinoid and cavernous sinus regions: measurement of critical structures relevant for surgical procedures. Clin Anat 8:3–9
Cushing H (1914) The Weir Mitchel Lecture. Surgical experiences with pituitary disorders. JAMA 63:1515–1525
De Divitiis E, Cappabianca P (2003) Endoscopic endonasal transsphenoidal surgery. Springer, Wien-New York
Frank G, Pasquini E, Mazzatenta D (2001) Extended transsphenoidal approach. J Neurosurg 95(5):917–918
Hardy J (1969) Transsphenoidal microsurgery of the normal and pathological pituitary. Clin Neurosurg 16:185–217
Hart CK, Theodosopoulos PV, Zimmer LA (2009) Anatomy of the optic canal: a computed tomography study of endoscopic nerve decompression. Ann Otol Rhinol Laryngol 118(12):839–844
Jho HD (2001) Endoscopic endonasal approach to the optic nerve: a technical note. Minim Invasive Neurosurg 44(4):190–193
Jho HD (2001) The expanding role of endoscopy in skull base surgery. Indications and instruments. Clin Neurosurg 48:287–305
Koc K, Anik I, Altintas O, Ceylan S (2008) Endoscopic optic nerve decompression for idiopathic intracranial hypertension in two cases: case report. Minim Invasive Neurosurg 51(2):72–75
Lang J, Kageyama I (1990) Clinical anatomy of the blood spaces and blood vessels surrounding the siphon of the internal carotid artery. Acta Anat 139:320–325
Laws ER, Sheehan JP (2006) Pituitary surgery––a modern approach. In: Grossman AB (eds) Frontiers of hormone research, vol 34, Karger, London
Li J, Wang J, Jing X et al (2008) Transsphenoidal optic nerve decompression: an endoscopic anatomic study. J Craniofacial surg 19:1670–1674
Maurer J, Hinni M, Mann W, Pfeiffer N (1999) Optic nerve decompression in trauma and tumor patients. Eur Arch Otorhinolaryngol 256(7):341–345
Patrocínio JA, Patrocínio LG, Júnior FB, da Cunha AR (2005) Endoscopic decompression of the optic nerve in pseudotumor cerebri. Auris Nasus Larynx 32(2):199–203
Rhoton A (2002) The orbit. Neurosurgery 51(4 Suppl):S303–S334
Rhoton A (2002) The sellar region. Neurosurgery 51(4 Suppl):S335–S374
Salcman M, Heros R, Laws ER, Sonntag V (2002) Kempe’s Operative Neurosurgery. Springer Verlag, Berlin
Schmidek Sweet (2000) Operative neurosurgical techniques. Section VI: pituitary tumors. Saunders, New York
Yuen AP, Kwan KY, Chan E, Kung AW, Lam KS (2002) Endoscopic transnasal orbital decompression for thyrotoxic orbitopathy. Hong Kong Med J 8(6):406–410
Zervas NT (1980) Reflections on the surgery of the pituitary. Clin Neurosurg 27:124–132
Acknowledgments
This work was supported by the Ricerca Corrente Funds of Fondazione Policlinico IRCCS, Milan.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Locatelli, M., Caroli, M., Pluderi, M. et al. Endoscopic transsphenoidal optic nerve decompression: an anatomical study. Surg Radiol Anat 33, 257–262 (2011). https://doi.org/10.1007/s00276-010-0734-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-010-0734-1