Abstract
When patients report pain in the popliteal fossa upon knee extension, the pain is usually localized in the lower region of the popliteal fossa. However, some patients complain of pain in the upper region of the popliteal fossa as the knee is flexed, which motivated us to examine the role of the popliteal fascia as the retinaculum of the hamstring muscles. Thirty-four thighs from 19 Japanese cadavers were dissected. The popliteal fascia was defined as the single aponeurotic sheet covering the popliteal fossa. We found that the fascia acted as a three-layered retinaculum for the flexor muscles of the thigh and provided a secure route for neurovascular structures to the lower leg in any kinetic position of the knee joint. The superficial layer of the popliteal fascia covering the thigh was strongly interwoven with the epimysium of biceps femoris along its lateral aspect and with that of the semimembranosus along its medial aspect, ensuring that the flexor muscles remained in their correct positions. The intermediate layer arose from the medial side of biceps femoris and merged medially with the superficial layer. The profound layer stretched transversely between the biceps femoris and the semimembranosus. Moreover, we investigated the nerve distribution in the popliteal fascia using Sihler’s staining and whole-mount immunostaining for neurofilaments. The three-layered fascia was constantly innervated by branches from the posterior femoral cutaneous or saphenous nerve. The nerves were closely related and distributed to densely packed collagen fibers in the superficial layer as free or encapsulated nerve endings, suggesting that the fascia is involved in pain in the upper region of the popliteal fossa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Disorders that cause swelling in the popliteal fossa make knee extension painful because the fossa is a confined space (Moore et al. 2010). The pain is felt in the lower region of the popliteal fossa in conditions such as Baker’s cyst, posterior cruciate ligament injury, popliteus tendinitis, and popliteal artery entrapment syndrome (Shimojo 2002; Terayama et al. 1996). However, patients sometimes complain of pain in the upper and relatively superficial region of the popliteal fossa when the knee joint is flexed. In such cases, it is difficult to diagnose the pain caused by disorders of the flexor muscles or the knee joint. Physical treatment by pushing the hamstring muscles medially to loosen the tension on the popliteal fascia decreased pain in our knee flexure test (Fig. 1). This finding motivated us to morphologically examine the popliteal fascia as a retinaculum of the hamstring muscles and to examine the innervation of the fascia.
Knee flexure test (popliteal region of left thigh viewed from the dorsal side). Patients complained of pain in the upper and relatively superficial region of the popliteal fossa as the knee was flexed. Pushing the hamstring muscles in a medial direction (arrows) to loosen the tension on the popliteal fascia decreased the pain in the flexed knee position
The deep fascia is chiefly composed of fibers that are arranged compactly with a high degree of directional regularity. The parallel fibers of one layer are set at an angle to neighboring layers. In the limbs (where the deep fascia is well developed), many collagen fibers are longitudinally arranged whereas others encircle the limb, binding the longitudinal fibers together into a tough, inelastic sheath for the musculature. There is a particularly powerful band of deep fascia in the thigh to transmit the tension of the tensor fascia latae (Warwick and Williams 1973). Stecco et al. (2009) stated that the fascia lata is independent of the underlying muscle; they are separated by the epimysium and a layer of loose connective tissue. A few strong intermuscular septa originate from the inner surface of the fascia lata and extend between the muscle bellies, dividing the thigh into different compartments. The popliteal fascia has been defined as a single aponeurotic sheet that covers the popliteal fossa because the retinaculum retains the hamstring tendons passing deep to it; it is continuous with the fascia lata superiorly and the crural fascia inferiorly to protect neurovascular structures within the popliteal fossa (Moore et al. 2010). Iguchi (1957) examined the thickness, texture, and direction of the collagenous fibers of the popliteal fascia, and reported that the longitudinal fibers thicken along the lateral side of the fascia whereas other fibers that are set at an angle run mostly from craniolateral to caudomedial. The term “retinaculum” describes a localized transverse thickening in the deep fascia that is attached at both ends to local bony prominences. Retinacula retain tendons passing deep to them that would otherwise be dragged or bowed out of position by the activities of their muscles (Warwick and Williams 1973). However, our study revealed that the popliteal fascia has a three-layered structure in the popliteal fossa that acts as multilayered kinetic retinaculum for the flexor muscles of the thigh and provides a secure route to the lower leg for the neurovascular structures enclosed in thick adipose tissues when the knee joint is in any kinetic position.
Concerning the fascial innervation, Benjamin (2009) suggested in his review that the “Fascia is richly innervated, but it is difficult to decide from the literature whether a particular layer of deep fascia is itself innervated or whether the nerve fibers lie on its surface or in the areolar or adipose tissue associated with it,” and then concluded that “Our understanding of fascia innervation is still incomplete.” Here, we used Sihler’s method and whole-mount immunostaining of neurofilaments to examine the macroscopic and microscopic anatomy, respectively, of the innervation of the three-layered popliteal fascia.
Materials and methods
We investigated 34 popliteal fasciae (16 right-sided and 18 left-sided) obtained from 19 Japanese cadavers (8 males and 11 females with ages ranging from 69 to 96 years at death) in the Department of Anatomy, Iwate Medical University. This study was conducted under standard consent and with the ethical approval of Iwate Medical University.
Gross anatomical structures of the popliteal fossa
In 18 thighs from nine cadavers, the fascia, flexor muscles, and nerves were carefully dissected. The thigh was cut transversely at the level of the gluteal fold proximally and at the level of the head of the fibula distally, and the femur was removed. To examine the relative positions of the flexor muscles, fascia, and neurovascular structures, two thighs (one flexed and one extended) were cut transversely approximately 6 cm proximal to the knee joint and the cross-sections were compared.
Adipose tissue removal using detergent and xylene
The popliteal fossa was filled with thick adipose tissue, which limited precise observation of the architecture of the fascia, particularly of the profound layer of popliteal fascia that comprised fine and delicate fibers. To exclude artifacts during gross anatomical preparation, the adipose tissue was removed from one left thigh as follows (Fig. 2e). The skin and subcutaneous tissues of the thigh were removed. The distal third of the thigh was soaked in a solution of 50 % alkaline hard detergent (Globe Environmental Protector Co., Ltd. Koriyama City, Fukushima, Japan) and water (40 °C) for 2 days. Next, it was washed with hot running water (40 °C) overnight, dehydrated with 50, 70, 90, and 100 % alcohol (for 2 days at each alcohol concentration), and soaked in a mixture of alcohol and xylene and then 100 % xylene for 2 h. The specimen was returned to 100, 90, 70, and then 50 % alcohol in order (for 1 day at each alcohol concentration) and preserved in 30 % alcohol for observation.
The SF, IF, and PF in the left thigh, with explanatory diagrams to demonstrate the anatomical architecture of each layer with respect to the hamstring muscles (a, b, and c are viewed from the dorsal side; in d and e, the femur has been removed and the specimen is viewed from the ventral side). a The SF was interwoven with the epimysium of the BFL (black arrow) along its lateral aspect (black arrowheads). The SF was detached from the lateral intermuscular septum of the thigh and turned over medially to show the connection. b The SF was interwoven with the epimysium of SM along its medial aspects (black arrowheads). The SF was cut along the medial aspect of the thigh and turned over laterally to show the connection. Black arrow indicates the ST tendon. c The SF was cut along the median line in which the SF merged with the IF (white arrowheads) and turned over to show the IF arising from the medial side of the BFL (black arrowheads). d The IF arising from the medial side of the BFL runs transversely to merge into the SF, and covers SM medially. The PFC and PFC-b (*) descend into thin adipose tissue between the SF and IF. The ST was removed in this specimen. e Fine and delicate fibers of the PF stretched transversely between the BF and SM. To exclude artifacts from gross anatomical preparation, adipose tissue was removed using xylene from this specimen (see “Materials and methods”)
Sihler’s staining method
To precisely examine the distributions of nerves to the fascia for eight thighs from four cadavers, the superficial and intermediate layers of the popliteal fascia were dissected and stained with Sihler’s solution, as described by Sekiya et al. 2005. After the staining, they were washed in running water while the color density of the stained nerves was checked. They were then soaked in 0.05 % lithium carbonate for neutralization until the nerves turned from purple to blue. The specimens were gradually cleared with 40, 60, and 80 % glycerol and kept in a cool dark place in 100 % glycerol. The collagen fibers and the nerves running in the fascia were observed using a stereoscopic microscope (Leica MZ16FA).
Whole-mount neurofilament immunostaining
To visualize nerve endings in the fascia, immunostaining was performed on one right thigh as follows. The superficial layers of the fascia were fixed in 10 % formaldehyde, placed in 15 % and then 30 % sucrose phosphate-buffered saline (PBS) solution, frozen (−20 °C), and then defrosted. They were washed with 0.1 M Tris buffer saline (TBS) for 15 min (three times) and distilled water (DW) for 20 min (twice), placed in 100 % methanol for 20 min (twice) and in H2O2 methanol (methanol 90 ml + H2O2 10 ml), and then washed with DW for 20 min (twice) and TBS for 2 h (twice). The specimens were placed in 10 % normal goat serum for 2–6 h at room temperature and then overnight at 4 °C, and then placed in RT97 monoclonal antibody (kindly donated by Professor B.H. Anderton, London) for 1–2 days at 4 °C and subsequently at room temperature for 1 h. Next, they were washed with TBS for 15 min (three times) before being placed in a secondary antibody (peroxidase-labeled affinity-purified goat anti-mouse IgG, KPL) overnight (4 °C), in PBS for 30 min (eight times), and in TBS for 30 min (four times). After diaminobenzidine color development, the specimens were washed with DW and cleared with 30, 60, and 80 % glycerol for observation.
Cross-sections
Thighs were cut transversely approximately 6 cm proximal to the knee joint and at the level of the knee joint distally, and the extensor muscles were removed (two thighs from two cadavers). The specimens were decalcified in 10 % EDTA-4Na solution (1 month), dehydrated with ethanol, and embedded in paraffin. They were sectioned at 5 μm using a sledge microtome (18E-M-0825: Jung) with a cryofilm transfer method (Kawamoto’s method: Leica, Japan) and stained with hematoxylin and eosin for general histology and azan stain for collagen fibers. The specimens were observed using a stereoscopic microscope (Leica MZ16FA). We also sectioned at 3 μm the superficial and the intermediate layers of the popliteal fascia from two thighs from one body, and performed Masson’s trichrome staining for collagen fibers. The specimens were observed using a light microscope (Olympus BX51).
Results
Three-layered architecture of the popliteal fascia
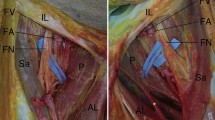
In the popliteal region, tough fascia covered all the flexor muscles of the thigh and was continuous with the lateral and medial intermuscular septa. The fascial covering on the thigh was strongly interwoven with the epimysium of the long head of the biceps femoris muscle (BFL) along its lateral aspect (Fig. 2a) and with that of the semimembranosus muscle (SM) along its medial aspect (Fig. 2b). Thick collagen fibers arranged in a row ran obliquely from craniolateral to caudomedial in the fascia (Fig. 3Ia–Ic). The fascia constrained the position of the biceps femoris muscle (BF) and the SM when the knee joint was at any kinetic position. Moreover, it held the long tendon of the semitendinosus muscle (ST) in the concave surface of the SM, acting as the retinaculum when the knee was extended (Figs. 4a, 5a). Here, we abbreviate this superficial fascia to “SF.” Another deeper fascia in which thick collagen fibers arranged in a row ran transversely (Fig. 2c, d) or obliquely from caudolateral to craniomedial was also recognized. This fascia arose from the medial side of the BF and merged into the SF medially (Figs. 2, 4, 5, 6a). We abbreviate this intermediate fascia to “IF.” Thin adipose tissue filled the space between the SF and IF. Beneath the IF, the popliteal fossa was filled with thick adipose tissue (Fig. 4). There were delicate fibers stretching transversely between the BF in a ventromedial aspect and the SM in a lateral aspect (Fig. 2e). We abbreviate this profound fascia to “PF.” The three-layered structure of the popliteal fascia was macroscopically and microscopically observed in the cross-section of the thigh, as shown in Fig. 4. The SF, IF, and PF distally converged into the single-layered crural fascia.
I Collagen fibers and cutaneous nerves of the SF in the left thigh. Ia Thick fibers running obliquely from craniolateral to caudomedial and the descending PFC and its branch (PFC-b). The specimen, stained with Sihler’s solution, was viewed from the ventral side. Ib In two of 16 cases, the PFC lacked the branch distributing to the SF, IF, and PF. To substitute for the PFC, the SA branched a ramus (*) medially to the popliteal fascia. Viewed from the ventral side. Ic Capillaries (white arrowheads) and the PFC-b forming a pseudo-ganglion (white arrow) interwoven among parallel collagen fibers. The specimen, stained with Sihler’s solution, was viewed from the dorsal side. II Whole-mount immunostained specimens of neurofilaments. IIa Free nerve endings (white arrows) to the collagen fibers and IIb encapsulated nerve endings (white arrows) in the connective tissue on the collagen fibers. IIc Cross-sections of densely packed collagen fibers (blue), and blood capillaries and nerve filaments (red) in the SF (stained with Masson’s trichrome)
Relative positions of flexor muscles and layers of the popliteal fascia in the left thigh viewed from the distal side. a The left thigh was cut transversely 6 cm proximal to the distal end of the Fe, and the extensor muscles were removed. The SF, IF, and PF are colored pink. The SF covered the BF laterally and the ST, SM, GRA, and SAR medially, and finally attached to the Fe by the lateral (LIS) and medial (MIS) intermuscular septa of the thigh. b Closer view of the black frame in Fig. 4a. The IF (white arrowheads) arising from the medial side of the BFL merged into the SF (black arrowheads) laterally. The PF (black arrows) stretched between the BF and SM. c Cross-section stained with azan (magnified view of the pink frame in Fig. 4a). The spaces between the SF and IF and the IF and PF were filled with thin and thick adipose tissue, respectively. The PFC-b was located in thin adipose tissue between the SF and IF. The neurovascular structures for the lower leg were enclosed within thick adipose tissue beneath the delicate PF in the deep area of the popliteal fossa
Semi-schematic drawings allowing the relative positions of muscles, neurovascular structures, and the SF, IF, and PF in cross-sections of the extended (a) and flexed (b) left thigh to be compared. Both thighs were cut transversely about 6 cm proximal to the distal end of the Fe and viewed from the craniodorsal side. Parallel black lines over the SF indicate the direction of collagen fibers
a Schematic drawing of the three-layered popliteal fascia and flexor muscles in the extended left thigh viewed from the cranioventral side. The SF connected with the BFL laterally and the SM medially (black arrowheads). The IF arising from the medial side of the BF (black arrowhead) merged with the SF medially and held the ST tendon in the concave surface of the SM, thus acting as a retinaculum. The PF stretched between BF and SM (arrows). The PFC and PFC-b mostly descended in thin adipose tissue between the SF and IF. b Schematic drawing of flexor insertions in the extended left thigh viewed from the dorsal side. The tendon of BF was attached to the head of the Fi and the SM inserted into the Ti and the lateral condyle of the Fe. The main part of the tendon attached to the tubercle on the posterior aspect of the medial condyle of the Ti, and the additional attachments to the medial margin of the condyle of the Ti. Various fascicles passed upward and laterally to the intercondylar line and lateral condyle of the Fe, forming the oblique popliteal ligament of the knee joint. The insertion of the ST was attached to the upper medial surface of the Ti and passed downward in part of the groove on the back of the SM
We investigated how the SF, IF, and PF act as the retinacula which ensures that the flexor muscles remain in the same position whether the knee joint is extended, semi-flexed, or flexed. When the leg was extended, the flexors of the thigh contracted moderately as antagonistic muscles for the quadriceps femoris muscle to prevent overextension of the knee joint (Fig. 5a). The contraction of SM strained the posterior ligament of the knee joint to prevent lateral rotation of the leg, and the BF prevented medial rotation of the leg. The flexors of the thigh kept the knee joint straight when the leg was extended. As described above, the SF covering the flexor muscles was interwoven with the BFL laterally and the SM medially (Fig. 2a, b), and the IF arising from the medial side of the BF merged medially into the SF (Fig. 2c, d). The constriction of the SM and BFL strained the thick collagen fibers of the SF laterally and those of the IF medially. Furthermore, it held the long tendon of the ST in the concave surface of the SM, thus acting as the retinaculum. Extending movements of the flexor muscles and knee joint compressed the thick adipose tissue enclosing neurovascular structures within the popliteal fossa. The adipose tissue (corpus adiposum popliteum; see the “Discussion”) bulged the taut SF and IF in a dorsal direction (Fig. 5a). The fibrous PF stretched transversely between the BF and SM to constrain muscles and neurovascular structures in the deep part of the popliteal fossa (Fig. 5a, b).
As the quadriceps femoris muscle relaxed, the flexor muscles of the thigh semi-flexed the knee joint, and the BF could act as a lateral rotator and the SM and ST as medial rotators of the leg. The BF tendon could be felt laterally and the ST tendon was more conspicuously medial to the popliteal fossa. When the knee joint was semi-flexed, the SF, IF, and PF were relatively loose and the popliteal fossa became flat.
When the knee was deeply flexed (Fig. 5b), the SM and ST muscles acted intensely as flexors and medial rotators and BF as a flexor and lateral rotator. According to the flexion angle of the knee joint, the SM tendon shifted dorsomedially and strained the SF in the same direction. The ST tendon attached to the upper part of the medial surface of the tibia stood out prominently along the lateral side of the SM tendon, and elevation of the long and thin ST tendon pulled the SF and the SM in a dorsal direction. The BF tendon attached to the head of the fibula protruded dorsally along the lateral side of the thigh, and the SF was pulled dorsolaterally by the elevation of the BFL tendon. In the deep flexed knee position, the IF pulled SF ventrolaterally because the IF stretched between the medial side of the BF and SF. The high tension in the ventral direction caused by the IF created a deep depression over the SF. The PF, consisting of delicate fibers, kept the BF and SM in their correct positions and, when the knee joint was deeply flexed, also provided a secure route to the lower leg for the neurovascular structures enclosed within thick adipose tissue (Fig. 5b).
Innervation of the popliteal fascia
We examined the relative position of the posterior femoral cutaneous nerve (PFC) and its distribution to the SF, IF, and PF in the 16 cases. The PFC descended the back of the thigh superficial to BFL and deep to the fascia lata. It medially and laterally branched into cutaneous rami, which pierced the fascia lata to distribute to the flexor aspect of the thigh (Fig. 3Ia). In 12 of 14 cases, the PFC descended inferiorly in the thin adipose tissue between the SF and IF and branched into a few rami that distributed to the SF, IF, and PF. The ramus to the SF was interwoven with the parallel thick fibers, adopting a flat and elliptic pseudoganglion-like form (Fig. 3Ic), before the nerve ended as a free nerve in the collagen fibers or as an encapsulated nerve in the connective tissue on the collagen fibers (Fig. 3IIa–c). In two of 14 cases, the PFC descended in the thick adipose tissue between the IF and PF. In those cases, the rami from the PFC pierced the IF to distribute to the SF. In two of 16 cases, the PFC totally lacked the ramus that distributes to the SF, IF, and PF: however, the saphenous nerve (SA), which descends on the lateral side of the femoral artery and enters the adductor canal, had a ramus distributing to the three-layered popliteal fascia from the medial thigh (Fig. 3Ib).
Discussion
Three-layered architecture of the popliteal fascia
As shown in Fig. 6b, the BF gradually narrows down to a tendon, and the main part of the tendon attaches to the head of the fibula. The SM is dorsomedial in the thigh and narrows to a tendon that gives off certain fibrous expansions to the back part of the outer condyle of the femur forming part of the posterior ligament of the knee joint, to the fascia that covers the popliteus muscle, and to the internal ligament of the knee joint (Warwick and Williams 1973). The ST ends a little below the middle of the thigh in a long, rounded tendon, which lies in the concave surface of the SM (Figs. 4a, 5a). The tendon is attached to the upper part of the medial surface of the tibia behind the attachment of the sartorius muscle (SAR) and below that of the gracilis muscle (GRA in Fig. 6b). As summarized in Fig. 6a, the SF covering the flexor muscles is interwoven with the BFL laterally and with the SM medially, and the IF originating from the medial side of the BF merges into the SF medially to keep the BF and SM in their correct positions regardless of the kinetic position of the knee joint. However, the ST does not have such a strong connection with the SF and IF. When the knee joint was deeply flexed, the long and thin ST tendon stood out prominently along the medial side of the thigh to the upper part of the tibia and lifted the SF and SM in a dorsomedial direction. The BF tendon stood out along the lateral side of the thigh to the head of the fibula, lifting the SF in a dorsolateral direction. Lang and Wachsmuth (1972) identified the flexor muscles, tendons, and bones which cause a fossa, sulci, or bulges on the skin surface of the popliteal region as the knee joint is flexed and extended, and also noted that rich adipose tissue in the popliteal fossa (corpus adiposum popliteum) bulged the skin in a dorsal direction when the knee was extended. We have shown in this work how the SF, IF, and PF are positioned in relation to the muscles, tendons, and bones in such a way that they dictate the position and direction of movement of each hamstring muscle, regardless of the kinetic posture of the knee joint. We have also provided anatomical information on how the SF, IF, and PF provide a secure route to the lower leg for the neurovascular structures enclosed in thick adipose tissues, and the mechanisms (involving the SF and IF) that lead to a deep depression in the skin in the flexed knee posture and a dorsal bulge in the extended knee posture (Fig. 5a).
Innervation of the popliteal fascia
As described in the “Introduction,” some patients have complained of pain in the upper and relatively superficial region of the popliteal fossa. However, the particular structures that caused this pain could not be identified, except for the fascia in this region. Our physical treatment to loosen the tension on the fascia decreased the pain as the knee joint was flexed. Benjamin (2009) reviewed several reports and stated that the fascia is richly innervated with abundant free and encapsulated nerve endings, but it is unclear whether a particular piece of deep fascia is itself innervated or whether the nerve fibers lie on its surface or in areolar or adipose tissue associated with it. Sanchis-Alfonso and Rosello-Sastre (2000) performed immunohistochemical analysis to understand the contribution of the lateral retinaculum to anterior knee pain syndrome. They described that the free nerve endings are slowly adapting receptors that mediate nociception and are activated in response to tissue deformation resulting from abnormal tensile and compressive forces in the fascia. Fairclough et al. (2006) re-evaluated the clinical anatomy of the lateral knee in order to gain a deeper understanding of iliotibial band syndrome, and noted that the region between the iliotibial band and the femur was filled with a highly vascularized and richly innervated adipose tissue that contained Pacinian corpuscles and bundles of myelinated and non-myelinated nerve fibers. They suggested that nerve fibers and Pacinian corpuscles in the fat were associated with the pain of iliotibial band syndrome. Both authors predicted that there would be greater innervation in the areolar connective tissue than in the fascia itself because intense mechanical stress on the fascia would damage the nerves. However, Stecco et al. (2009) showed that nerve fibers are closely related to the densely packed collagen fibers in the fascia lata. As Benjamin (2009) described, “Our understanding of fascia innervation is still incomplete, and it is likely that there are regional differences of functional significance.”
The dispute over fascial innervation motivated us to examine the nerve distribution to the three-layered fascia in the popliteal fossa. The SF, IF, and PF were innervated by a branch from the PFC descending in the adipose tissue between the SF and IF (in 12 of 14 cases) or the IF and PF (in 2 of 14 cases). In 2 of 16 cases, the PFC lacked this branch, but the SA compensated for the PFC, distributing a branch to the popliteal fascia from the medial side of the thigh. The rami from the PFC were intimately related to the SF and the medial side of the IF merged with the SF, in which the nerves were textured with the parallel collagenous fibers and sometimes formed pseudoganglions. The rami ended as free or encapsulated nerve endings in the collagen fibers of the fascia in the SF. Our findings demonstrate that the fascial nerves are closely related to the densely packed collagen fibers and lie within the fascia itself in the popliteal region.
To summarize, we found that the popliteal fascia has a three-layered structure that acts as a kinetic retinaculum for the flexor muscles of the thigh, and that the innervation of the fascia suggests a possible relationship to popliteal pain. Furthermore, this multilayered architecture provides a secure route to the lower leg for the neurovascular structures enclosed in thick adipose tissue, regardless of the kinetic position of the knee joint.
Abbreviations
- Ne:
-
Nerve ending
- PA:
-
Popliteal artery
- PFC:
-
Posterior femoral cutaneous nerve
- PFC-b:
-
Branch of posterior femoral cutaneous nerve
- PV:
-
Popliteal vein
- SA:
-
Saphenous nerve
- SA-b:
-
Branch of saphenous nerve
- SCI:
-
Sciatic nerve
- Fe:
-
Femur
- Fi:
-
Fibula
- Ti:
-
Tibia
- IF:
-
Intermediate layer of popliteal fascia
- PF:
-
Profound layer of popliteal fascia
- SF:
-
Superficial layer of popliteal fascia
- BF:
-
Biceps femoris muscle
- BFL:
-
Long head of biceps femoris muscle
- BFS:
-
Short head of biceps femoris muscle
- GRA:
-
Gracilis muscle
- LIS:
-
Lateral intermuscular septum of thigh
- MIS:
-
Medial intermuscular septum of thigh
- SAR:
-
Sartorius muscle
- SM:
-
Semimembranosus muscle
- ST:
-
Semitendinosus muscle
References
Benjamin M (2009) The fascia of the limbs and back—a review. J Anat 214:1–18
Fairclough J, Hayashi K, Toumi H et al (2006) The functional anatomy of the iliotibial band during flexion and extension of the knee: implications for understanding iliotibial band syndrome. J Anat 208:309–316
Iguchi S (1957) Kashi no Kinmaku ni tuite (Deep fascia of lower limb). Kumamoto Med 41:143–172 (in Japanese)
Lang J, Wachsmuth W (1972) Praktische anatomie: bein und statik. Springer, Berlin, pp 206–222
Moore KL, Dalley AF, Agur AMR (2010) Chapter 5: Lower limb. Clinically oriented anatomy, 6th edn. Lippincott Williams & Wilkins, Philadelphia, pp 569–586
Sanchis-Alfonso V, Rosello-Sastre E (2000) Immunohistochemical analysis for neural markers of the lateral retinaculum in patients with isolated symptomatic patellofemoral malalignment. a neuroanatomic basis for anterior knee pain in the active young patients. Am J Sports Med 28:725–731
Sekiya S, Suzuki R, Miyawaki M, Chiba S, Kumaki K (2005) Application of the modified Sihler’s stain technique to cadaveric peripheral nerves after medical students’ dissection course. Kaibogaku Si 80:67–72 (in Japanese with English abstract)
Shimojo H (2002) Overuse syndrome of the knee joint. MB Orthop 15:10–16 (in Japanese)
Stecco A, Macchi VM, Masiero S et al (2009) Pectoral and femoral fasciae: common aspect and regional specializations. Surg Radiol Anat 31:35–42
Terayama K, Kataoka O, Torisu T (1996) Approach to the pain in orthopaedics: the thigh and the knee. Nankodo, Tokyo, pp 15–26 (in Japanese)
Warwick R, Williams PL (1973) Gray’s anatomy, 35th edn. Longman, Edinburgh, pp 561–571
Acknowledgments
The authors are grateful to Dr. Shin-ichi Sekiya of Niigata College of Nursing for advice on Sihler’s staining, and Dr. Koujirou Touyama of Iwate Medical University School of Medicine for advice on neurofilament immunostaining (RT97 monoclonal antibody was kindly donated by Dr. B.H. Anderton, London). We also express gratitude to Sadanari Takahashi and Yukie Takahashi of Iwate Medical University School of Medicine for technical support. We dedicate this article to the late Dr. Masaharu Horiguchi (Department of Anatomy, School of Medicine, Iwate Medical University), who provided suggestions and encouragement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Satoh, M., Yoshino, H., Fujimura, A. et al. Three-layered architecture of the popliteal fascia that acts as a kinetic retinaculum for the hamstring muscles. Anat Sci Int 91, 341–349 (2016). https://doi.org/10.1007/s12565-015-0306-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12565-015-0306-x