Abstract
Increased water scarcity demands more efficient use of water in the agricultural sector which is the primary consumer of water. Precise determination of irrigation requirements based on specific crop parameters is needed for accurate water applications. We conducted a 4-year study on almond evapotranspiration using a large weighing lysimeter. Tree canopies changed from 3 to 48 % ground cover during the course of the study. Sap flow measurements made on the lysimeter tree provided a continuous record of tree transpiration. We propose to use the daily fraction of photosynthetically active radiation intercepted by the canopy (fIRd) as a predictor of almond orchard maximum transpiration. The transpiration coefficient (T/ET o or K T ) was related to the fIRd of the last two years, and the ratio between fIRd and K T stayed more or less constant around a value of 1.2. Such value extrapolated to the size of a mature orchard with 85 % intercepted radiation gives a K T of around 1.0, a number above the standard recommendations, but fully compatible with the maximum K c values of 1.1–1.15 recently reported.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Population growth and economic development are some of the major causes of the increased water scarcity in many world areas. Mounting pressure on the available water resources is occurring as the global temperature increases and there is a risk of an augment in the intensity and duration of droughts in the future (Stocker et al. 2013). Within this context, efficient use of water is of paramount importance, in particular in the agricultural sector, the primary user of developed water. As more efficient methods of irrigation are being adopted worldwide, the precise estimation of crop water requirements is an important objective (Lenton 2014). The established method of computing crop water requirements follows the crop coefficient approach, as described in the FAO Monographs 24 and 56 (Doorenbos and Pruitt 1977; Allen et al. 1998). Those crop coefficients (K c) relate empirically the water consumption of a healthy, well-watered crop to the reference evapotranspiration (ET o) which is the consumption of a reference grass crop under the same climatic conditions. Values for the K c’s of different crops have been recommended for the different growth stages. In the case of fruit tree crops, a wide range of K c values were proposed by Allen et al. (1998) based on the original values proposed earlier by Doorenbos and Pruitt (1977). The recommended maximum (mid-stage) K c values for different fruit tree species, without a cover crop, ranged between 0.65 and 1.10, and in the case of almond orchards, the K c values for initial, medium and final stage were 0.40, 0.90 and 0.65, respectively (Allen et al. 1998). Such recommendation indicates that mature almond orchard ET at full cover would be <ET o (in fact, recommended mid-season K c values ranged from 0.8 to 0.95, depending on air humidity and windspeed; Doorenbos and Pruitt 1977). However, there have been recent reports that propose higher mid-stage K c values than those recommended in Allen et al. (1998) for almonds. Girona (2006) and Sanden (2007) have proposed almond K c values close to 1.05 for the summer. One explanation for this discrepancy is that the intensification of almond production that has occurred over the last decades, based on increases in tree density and minimal pruning, has led to greater radiation interception than what was intercepted by the orchards where the original K c values were determined. Recent work focused on measuring almond ET using the eddy covariance technique has determined a maximum K c value of 1.12 in a high-yielding (4.3 t ha−1) almond orchard in Australia irrigated with microsprinklers (Stevens et al. 2012). Sanden et al. (2012) proposed a maximum K c value of 1.15 for high-yielding almond orchards (4.0 t ha−1), even though yields were the same than those obtained when irrigation was applied following the previous K c values of Sanden (2007).
Given that crop coefficients include not only transpiration (T) but also evaporation from the soil (E), which might vary substantially depending on the frequency and method of irrigation, it is important to separate the two components of the ET process. In Allen et al. (1998), an approach to separate the E and T components of ET is referred to as the dual crop coefficient approach, where K c is separated into a basal crop coefficient (K cb) and an evaporation coefficient (K e). K cb is nearly equivalent to the crop transpiration divided by the ET o (assuming E is negligible when the soil surface is dry), and should be independent of irrigation system and frequency, making the K cb more applicable to various orchards differing in features affecting the E component of ET.
In the case of tree orchards, a year–year evolution of K c or K cb has to be determined because the canopy changes dramatically from young to mature trees. Doorenbos and Pruitt (1977) proposed K c values for canopies covering only 20 and 50 % of the ground. They recommended a reduction in maximum K c values by 25–35 % and by 10–15 %, respectively. Allen et al. (1998) utilize an adjusted coefficient (K c adj) to reduce a basal crop coefficient that represents full-cover conditions (K c full), when the coverage of the ground by the crop is incomplete. This K c adj, based on ground cover (GC) and tree height, takes values of 0.40 and 0.89 for 20 and 50 % cover, respectively. More recently, Allen and Pereira (2009) modified that approach and substituted the K c adj for a density coefficient (K d) that yields values of 0.30 and 0.78 for 20 and 50 %, respectively, quite different from the recommendations of Allen et al. (1998). In summary, there is substantial uncertainty on how to adjust the K c or K cb values to situations of incomplete GC in developing tree crops such as almonds.
Fereres et al. (1982) measured the evapotranspiration of drip-irrigated young almond trees (1–3 year old) and related the values to the degree of GC of the trees (horizontal projection of the shade). The measurements were reported as percentages of the calculated ET of a mature orchard, assumed to have a GC of 60 % at the time. The empirical relation obtained has been widely used since that time to compute the water use of young trees in semi-arid climates (Castel 1997; Ayars et al. 2003). Recently, Fereres et al. (2012) have proposed a similar relation, based on experimental data from other several tree species, where the young tree ET is calculated as a percentage of mature orchards ET according to the degree of GC. However, the shaded area of a tree (GC) is a crude estimate of intercepted radiation as it does not take into account factors such as leaf area density (LAD), tree height, row orientation and canopy architecture, which varies depending on the species, variety and time of season. These factors influence the amount of intercepted radiation, which, for a given stomatal conductance, is the key factor affecting tree transpiration.
Actually, Green et al. (2003a) and Ayars et al. (2003) have shown that transpiration is directly related to the amount of intercepted radiation in apple and peach, and they found that the relationship was constant through the season. Pereira et al. (2007) found a single linear relationship between daily transpiration (T) and daily net radiation multiplied by leaf area index in apple, walnut and olive. However, Girona et al. (2011) found different relationships between midday fraction of photosynthetically active radiation intercepted (PAR) by the tree (fIRmd) and T in pear and apple and reported declines in K c after harvest without changes in canopy foliage. Goodwin et al. (2006) in peach and Consoli et al. (2006) in citrus related T to the daily integral of PAR intercepted radiation (fIRd) instead of relating it to fIRmd and proposed this methodology to improve the prediction of K c values from measurements of fIRd.
Recent studies have successfully predicted T from fIRd in apple (Auzmendi et al. 2011; Casadesus et al. 2011). Casadesus et al. (2011) highlighted the need to normalize the relationship for temperature as in some species with little stomatal response to high evaporative demand, the sensitivity to changes in ET o is more important than to variations in fIRd. Marsal et al. (2013, 2014) have used a simulation model (CropSyst) to predict K c from fIRd in apple and pear, although they proposed different relationships, depending on the species and on the stage of development. They defined a parameter, K cfc, which represents the maximum K c for a hypothetical crop that completely covers the soil surface, and then used fIRd to scale for tree size and shape.
The variations in the relation between T and fIRd might be related to differences in canopy conductance (g c). There are models that predict T based on the calculation of g c (Dekker et al. 2000). These models are generally complex with high input data requirements. Orgaz et al. (2007) developed a simplified g c model to reduce the input requirements and showed good performance in predicting T of olive trees. Villalobos et al. (2013) have extended the same approach to several fruit trees species where calibration was performed by measuring sap flow and daily IRd.
Given the uncertainties in the magnitude of the consumptive use requirements of almond orchards and their K c values discussed above, we conducted an investigation to measure the water use of young almond trees and to develop a generalized relationship between tree transpiration and the radiation intercepted by almond tree canopies.
Materials and methods
The experiment was conducted in a 5.5-ha almond orchard located at the Research Center of IFAPA-Alameda del Obispo, in Córdoba, Spain (37°52′N, 4°49′W). The orchard was planted in 2009 with ‘Guara,’ a late blooming, self-compatible cultivar (Felipe and Socias i Company 1987) grafted onto the GF-677 rootstock. Tree spacing was 6 × 7 m with rows E–W oriented. No crop cover was allowed to grow beneath the trees; vegetation control was done with periodic herbicide applications. A drip irrigation system was installed with a single drip line per row with 2.4 l h−1 emitters one meter apart; thus, every tree was irrigated with six emitters. In 2013, a new drip irrigation system was installed with two drip lines per tree row and 4 l h−1 emitters one meter apart along each drip line. Irrigation frequency was daily, and water requirements were calculated as ET o × K c times the reduction coefficient from Fereres et al. (2012) and then adjusted depending on the lysimeter data. If the lysimeter weight decreased with time, the application rate was increased accordingly. Weather data were collected using an automated weather station located over grass, located about 300 m apart from the orchard.
A large weighing lysimeter was constructed around the center of the orchard in 2009. The lysimeter consists of a stainless steel container 3 × 3 m and 2.15 m depth, supported by four load cells that measure continuously its weight with an accuracy of 0.5 kg, equivalent to 0.056 mm of water depth. Data are stored in a datalogger as 5-min averages and periodically downloaded to a computer. The complete design and construction of the lysimeter is described in Lorite et al. (2012). The orchard was planted in 2009, and one tree was planted in the center of the lysimeter container.
As the rest of the orchard, the lysimeter tree was irrigated with six emitters of 2.4 l h−1, located around the trunk until 2013, when the irrigation in the lysimeter was modified to install 24, 2 l h−1 emitters placed along four drip lines all over the lysimeter area. The goal of this change in the number of emission points was to achieve a wetted soil surface area that was similar to that of the trees outside the lysimeter, given that the area of the lysimeter (9 m2) was less than the area occupied by trees outside. Irrigation in the lysimeter was applied at night, simultaneously with the rest of the orchard, and was measured with a water meter. Weight loss during the daytime was considered equivalent to tree evapotranspiration (ET).
Regarding pruning, pest and disease control, the lysimeter tree was managed in the same fashion as the rest of the orchard. In addition to the standard fertilization program followed in the orchard, periodically, additional fertilizers were applied to the lysimeter to ensure that nutrients were not limiting tree growth due to the smaller soil volume explored by the lysimeter tree. Periodically, the lysimeter surface was covered with plastic to prevent evaporation. A thin layer of soil was placed on top of the plastic to avoid modification of the albedo around the tree. The weight loss during those periods was considered equivalent to tree transpiration (T).
Data collected on rainy days were not considered in the analysis, and in the case of heavy rains, data from the following days were also discarded.
Tree water status was monitored periodically in 2011, 2012 and 2013 by measuring stem water potential. Four neutron probe access tubes were installed in the lysimeter to monitor the soil water content profile. Tree nutritional status was monitored by leaf analysis once a year in 2012 and 2013.
Sap flow
The transpiration of the lysimeter-grown tree was measured with a sap flow system device developed and assembled at the IAS in Cordoba and described in Testi and Villalobos (2009). The system uses the compensation heat pulse (CHP) method plus the calibrated average gradient (CAG) technique (Testi and Villalobos 2009); the latter is used when low sap velocities (lower than 12 cm h−1) prevent the use of the former method or reduce its accuracy. To measure sap flow velocity, a set of probes and associated electronics connected to a logger are needed. One set of probes consists of one linear heater probe and two probes with four temperature sensors at different depths from the cambium to characterize the sap flow velocity profile. The probes were installed at an appropriate depth to measure sap velocity at 5, 15, 25 and 35 mm depths from the cambium. The heat pulse velocity is calculated by measuring the time when both temperature sensors are equilibrated. These values are then converted to sap velocity and are integrated first along the trunk radius (using the radial velocity profile curve given by the probe) and then around the azimuth angle (Green et al. 2003b) to convert them to sap flow. A full measurement cycle was performed every 15 min. The resultant sap flow integrated over the measurement period is considered equivalent to the transpiration of the tree. To account for sap flow azimuthal variability, previously reported in other species (López-Bernal et al. 2010), the measurement of sap flow transpiration (T sf) was calibrated using T data from the lysimeter. A calibration coefficient relating daily values of T sf to the daily values of lysimeter T was obtained for every set of T measurements in the lysimeter.
Tree canopy characterization
Canopy features were obtained by digital images of the lysimeter tree taken from at least four points of view. Every picture was scaled to real dimensions according to the reference length from a marked pole that was located near the trunk and in the same plane with the trunk as view from the photographer position. The measuring tool of a specialized software (AutoCAD-2012; Autodesk, San Rafael, CA, USA) was used to obtain tree height and horizontal and vertical radius of the canopy. An average value from the different positions of each of two dimensions was then calculated. The horizontal radii were used to calculate the percentage of GC in relation to tree spacing, and the canopy volume was obtained by approximating the tree shape to an ellipsoid.
Plant leaf area was estimated from the analysis of photographs of the tree shadow. The approach that we developed is based on the theory of transmittance (τ), that is, the probability of an unintercepted beam of radiation traveling from the beam’s source to the soil surface (Lang 1987).
where θ is the zenith angle, G(θ) is the foliage area projected perpendicular to the beam direction, LAD is LAD and S(θ) is the path length that the beam had to traverse through the canopy to reach the soil surface. Taking logarithm of both sides of the Eq. 1:
Considering the projection of the canopy divided into sectors, the LAD of a sector i corresponding to an area on the plane normal to the beam A ni is:
where PLA is the plant leaf area and is derived from Eq. 4:
when θ = 1 rad, G = 0.5 (Lang 1987) and then
A digital photograph RGB of the tree shadow is taken at the time of the day when θ = 1 rad, a time when G = 0.5. That photograph is then imported to ERDAS-IMAGINE (HEXAGON Geospatial, Stockholm, Sweden), an image processing software that allows users to work with pixels as a matrix of values. Then, a supervised classification of the image is carried out, by classifying every pixel as light or shadow. A value of 100 is assigned to light pixels and zero to shadow pixels. The next step is to degrade the image, that is, to create new larger pixels, containing a fixed number of old pixels. The value of the new created pixel is then the average of the original measured pixel values; therefore, it represents the percentage of non-shaded/shaded area which is equivalent to the transmissivity in that pixel. In a spreadsheet, the logarithm of the values of the new pixels is calculated and then averaged and the PLA calculated as Eq. 5. Finally, LAD was calculated by dividing plant leaf area by canopy volume.
For the validation of the method described above to compute PLA, all the leaves in four trees were collected and measured with the following procedure: At the beginning of October, before the onset of leaf fall, photographs of four trees in 2012 and of the lysimeter tree in 2013 were taken and processed following the approach described above. Afterwards on the same day, a sample of 200 leaves from each tree was collected and individual leaf areas were measured with an electronic area meter (Li-Cor LI-3100), to obtain the average area per leaf. Then, nets were placed to wrap the four tree canopies to collect all the leaves as they fell. At the end of autumn, all leaves from every tree were collected and weighted. A subsample of 500 leaves was weighted separately to obtain average leaf weight for the calculation of the number of leaves from the total weight. Finally, multiplying the number by the average leaf area of each tree, tree leaf area was calculated and the results compared against the values obtained using the photograph classification approach.
Relation between transpiration and intercepted radiation: canopy conductance model
The model developed by Mariscal et al. (2000), parameterized for almond trees, was used to derive the diurnally integrated fraction of fIRd by the tree canopy. The model inputs were planting arrangement, row orientation and tree shape. Besides, the model takes two parameters that are specific for a given species: LAD (m2 m−3) and the G-function (Ross 1981). The G-function defines the projection coefficient of unit foliage area on a plane perpendicular to the beam direction. Our procedure for its calculation in almond was obtaining the value of G for zero zenith angle (vertical) by measuring the vertical transmissivity with a Plant Canopy Analizer (model LAI-2000, Li-Cor biosciences, Lincoln, USA). In this way, we obtained LAD × G(0°), and as the value of LAD was known by the measurement of canopy volume and PLA, G(0°) could be derived. Then applying the ellipsoidal distribution (Campbell and Norman 1989), the value of G for 90° zenith angle is deduced. With these boundary values, G for any zenith angle is then obtained.
For the years 2012 and 2013, daily T/ET o (K T ) was divided by the calculated fIRd and plotted for both seasons.
The canopy conductance model of Villalobos et al. (2013) was used to derive transpiration from fRId and VPD as follows:
The bulk canopy conductance was calculated inverting the “imposed” evaporation equation (as in Villalobos et al. 2009) from the measured transpiration data. The parameters of the model (a and b) were calculated with daily T data of 2013.
Results
Evolution of tree size
Canopy volume increased from <1 m3 in spring 2010 to more than 50 m3 in autumn 2013 (Fig. 1). Ground cover increased from 3 % in 2010 to almost 50 % in 2013, when the branches of adjacent trees within rows were almost in contact. During the 4 years, tree height increased from 1.5 to 4.8 m.
Evolution of canopy volume and ground cover during the experimental years, 2010–2013. Circles indicate days of measurements. Periods without measurements in 2010 and 2011 were drawn following similar patterns of growth than for 2012 and 2013. Linear interpolation was used to complete the line between measurements
The comparison between measured tree leaf area and the value estimated by the photograph method is shown in Fig. 2. A linear relationship between estimated plant area and directly measured leaf area was found with good correlation (Fig. 2). When the trees were small, plant area estimated by the approach of digital image processing was very similar to the measured leaf area. However, as the size of the tree becomes bigger and the branch network increases in size and intercepts more radiation, the values obtained by the photograph method were higher than the measured leaf area obtained by collecting all the leaves.
Leaf area density (LAD) measurements are presented in Fig. 3. In 2012, the first measurement taken at the beginning of May gave a value of 1.0 m2 m−3, and as leaf area increased over time, LAD reached a value of 1.3 m2 m−3 in August. In 2013, in spite of the delay in canopy development, a similar pattern of LAD evolution was observed, and a maximum value of 1.4 m2 m−3 was reached in August. The extrapolation before and after the first and final measurement dates of every year (dashed lines) was drawn by field observation.
Evolution of transpiration
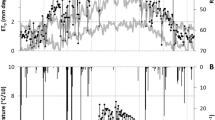
Figure 4 shows the evolution of transpiration measured in the lysimeter (T) for the four study years and also by sap flow (T sf) since 2012. ET o is also plotted for better interpretation of data. The period May 18–June 12, 2013, was discarded because the tree experienced some water stress due to a failure in the irrigation system of the orchard.
The seasonal evolution of transpiration is particularly evident in 2012 and 2013. At the beginning of the season, transpiration augments as ET o increases as a result of higher values of solar radiation, temperature and vapor pressure deficit, and as leaf growth proceeds. The maximum values of T are reached around mid-July when ET o is also maximum. Then, T decreases with time as ET o also diminishes.
In 2012 and 2013, maximum T values were 3.1 and 4.2 mm, respectively. In 2010 and 2011, T data collection during the stage of maximum T was limited. Nevertheless, according to the few values obtained around that period, maximum T probably reached 1 and 2.5 mm, respectively.
For each set of sap flow probes installed in the tree trunk, a calibration coefficient was calculated for every period when the lysimeter soil was kept covered. The calibration coefficients were nearly constant during 2012, but they increased slightly with time in 2013 (data not shown). Each year, the coefficients of the two probes followed the same evolution with time, but the absolute values were notably different, indicating azimuthal variations in sap velocity.
Figure 5 presents the transpiration coefficient (T/ET o or K T ) for 2012 and 2013 when continuous T data were collected by sap flow. In spring, K T increases as leaves grow (Fig. 5b). After complete development of the canopy, transpiration responds to daily variations in ET o, and K T remains relatively constant, although there was significant day-to-day variability in K T (Fig. 5). In summer, K T reached maximum values and those maximum values increased every year, from values of 0.13 in 2010 up to 0.60 in 2013. We observed that the stage of constant K T extended into the fall season, and K T started to decline only about the end of October in 2012 and in mid-October of 2013. This stage of decreasing K T in autumn was characterized by a fast decline in water consumption as the tree defoliated.
Relations between K T and fraction of intercepted PAR radiation
Using the more detailed T records in 2012 and 2013, the relation between K T and IR was examined in detail for the last 2 years of this work. Figure 6 shows the evolution of LAD, GC and fraction of intercepted PAR radiation (fIRd) as a function of time for 2012 and 2013. The initial value of GC in 2012 was 21 %, and the final value was 35 %. In 2013, the initial and final values of GC were 34 and 49 %. Thus, from the beginning of the 2012 season till the end of 2013, GC more than doubled, from 21 to 49 %.
The evolution of the calculated fIRd is also shown in Fig. 6. Two stages of increase in fIRd can be observed: one associated to increments of GC and LAD and a second stage at the end of August where fIRd continued to increase, even though GC and LAD were almost constant.
Figure 7 shows the time course of the ratio between the daily transpiration coefficient (K T ) and the daily fraction of fIRd. Combined data from 2012 to 2013 are presented, starting after DOY 140 (mid-May) to DOY 300 (early November). Approximately until the end of September (DOY 270), daily values of the ratio K T /fIRd oscillated around 1.2, indicating a relatively constant relation between the transpiration and the amount of intercepted radiation during that period.
In 2013, the ratio K T /fIRd started decreasing from around DOY 255, before the drop of K T experienced from DOY 280. In 2012, K T did not decrease before DOY 300; however, a slight decrease occurred in the ratio K T /fIRd.
As the development of GC and fIRd was similar during 2012 and 2013, the evolution of the ratio K T /GC (Fig. 8) was very similar to the evolution of the ratio K T /fIRd, except for the early canopy development period where GC data were collected but no data of LAD and thus of intercepted radiation were available. In both cases (Figs. 7, 8), the ratios declined gradually during the senescence period from DOY 270 to 300.
Canopy conductance, intercepted radiation and VPD
The calibration of the model of Villalobos et al. (2013) is shown in Fig. 9, obtained by plotting the ratio of fIRd/g c against the daytime values of VPD for 2013. A linear relationship was found with a R 2 of 0.87, an intercept of 1083 µE mol−1 and a slope of 1106 µE mol−1 kPa−1.
Discussion
The transpiration of an orchard responds to canopy development and to the evaporative demand, quantified as ET o. In deciduous orchards, after the tree has completely developed its foliage, T varies mainly according to ET o, the transpiration coefficient (T/ET o) is expected to change very slightly and steadily—if there is creation of new leaf area—until the start of defoliation in autumn (Figs. 5, 6). Since the beginning of this study, the maximum daily T increased from around 1 mm to more than 4 mm, as the tree canopy expanded from 3 to 50 % GC, revealing the importance of adjusting the irrigation amounts of young orchards to tree size.
The transpiration coefficient is a function of leaf area and needs to be scaled up as the orchard grows. We proposed the daily fraction of fIRd, as a predictor of the K T evolution with tree size, following earlier works (Ayars et al. 2003; Green et al. 2003a; Marsal et al. 2014).
The ratio between daily K T and the corresponding fIRd (K T /fIRd) oscillated around the value of 1.2 during all the irrigation season, from DOY 140 (mid May) to DOY 250 (early September) (Fig. 7). Remarkably, the ratio did not vary between the two study years, despite the important changes in tree canopy expansion that went from a GC percentage of 21 to 48 %. This result entails that it is possible to predict the water use of an almond orchard of any age and canopy size by estimating its fIRd. The measured ratio between K T and fIRd allows the calculation of orchard water use on a daily basis and seems a useful tool for irrigation scheduling in almond during most of the irrigation season, i.e., before it shows a reduction in the fall (Fig. 7).
After the harvest of 2012 (DOY 237), no change was detected neither in K T nor in its relationship to fRId. By contrast, Auzmendi et al. (2011) in apple and Girona et al. (2011) in apple and pear found that K c diminished after fruit removal at harvest. In our case, only at the end of the irrigation season (DOY 250–260) the ratio K T /fIRd decreased; this behavior occurred not only in 2012 when K T diminished, but also in 2013 when K T was constant until DOY 300 (Figs. 5, 7). One reason might be that the increment in fIRd in autumn, due to the decreased solar elevation angle, does not translate into higher tree transpiration because of leaf aging and concomitant low stomatal conductance. In any case, the decrease in the K T /fIRd ratio appeared well after harvest August 24, 2012 (DOY 237) (Fig. 7). Our results are in accordance with Ayars et al. (2003) and Pereira et al. (2007) who found constant relationships between transpiration and absorbed energy during the whole season in peach and in walnut, olive and apple, respectively. In our case, we did not find any reduction in water use after fruit removal; this fact could be explained by the appearance of new carbon sinks; in 2012, when the trees had very high fruit load, we observed substantially new shoot growth at the end of August near and after the date of harvest.
In 2013, from DOY 190 until 235, the ratio K T /fIRd slightly drops below the 1.2 value. This moment coincides with the latency period of shoot growth in summer and may be explained by the low fruit load of 2013, which probably decreased the transpiration rate of the tree.
According to the ratio K T /fIRd of 1.2 obtained in this study and assuming that the maximum relative value of fIRd is 85 % (given the need for mechanization, it is not feasible to have 100 % intercepted radiation in most orchard crops), the maximum K T would be 1.2 × 0.85 or 1.02. This K T value is higher than the 0.85–0.9 value for almond K c recommended by Allen et al. (1998). Other K c values proposed by Girona (2006), Stevens et al. (2012) and Goldhamer and Girona (2012) are more in line with the 1.02 K T value obtained in this study. In fact, if we assume an evaporation of about 10–15 % of ET c (common in orchards irrigated by microsprinklers, such as the ones used in the two previously mentioned studies), the agreement between our results on K T and the recently found K c values for almond orchards is excellent.
Our K T /fIRd ratio is comparable to the parameter K cfc of the CropSyst model (Stöckle et al. 2003). For apple, peach and pear, Marsal et al. (2014) obtained K cfc values of 1.5, which is higher than our findings for almond.
We also found a good relationship between GC and transpiration (Fig. 8). Although the transpiration responds physiologically to fIRd, not to GC, these two variables are correlated. The stability over time and tree size of the index K T /GC are similar to that of K T /fIRd (Fig. 8). Ground cover is much easier to measure than fIRd, and K T may be conveniently estimated from GC when it is not possible to measure or estimate fIRd trustfully. However, factors such as cultivar, LAD or pruning system may affect the relation between GC and fIRd; thus, the value of 1.2 of the ratio (Fig. 8) should not be considered fixed among different orchards or stable in time after events such as a change in the canopy architecture due to pruning. Beside this, on the early canopy development period, the ratio K T /GC increased as new leaves developed for a similar GC, and thus, a fixed value should not be used for the entire season. The results of this study should be useful in making precise irrigation recommendations for almond orchards, based on the amount of water transpired by the tree. Evaporation from the soil could be calculated separately using a model such as the one developed by Bonachela et al. (1999, 2001) that has performed successfully in olive orchards. It has to be also taken into account that one of the objectives of irritation of young almond trees is to quickly reach an adult size, as yields are strongly correlated with the canopy volume of the tree. Then, the irrigation recommendations derived from the methodology proposed in this work might be exceeded to promote root growth and thus canopy growth. The value of the ratio K T /fIRd proposed here applies only to well-watered trees without diseases.
In the assessment of transpiration based on fIRd, an alternative approach to the use of a fixed value that we propose together with ET o is the use of the Penman–Monteith equation coupled with a canopy conductance model. Using transpiration data of 2013, we parameterized the bulk canopy conductance model of Villalobos et al. (2013) (Eq. 6) (Fig. 9). The intercept a (Eq. 6) is proportional to the radiation use efficiency, and the value obtained for almond (a = 1083 µE mol−1) would indicate that it is a specie more efficient in radiation capture than orange, peach, apricot, apple and pistachio, and less efficient than walnut and olive (cf. Villalobos et al. 2013). The slope b is related to the response of the specie to changes in VPD, a high slope indicates little sensitivity to VPD. The value of b obtained for almond (b = 1106 µE mol−1) lies in between the high values of apricot, orange and olive (species very sensitive to VPD) and the low values of peach, walnut and pistachio, and very close to the value of apple (Villalobos et al. 2013).
In most lysimeter studies, K c fluctuates significantly from day-to-day, particularly in tree crops (i.e., Girona et al. 2011), suggesting that ET c responds to daily weather conditions somewhat differently than ET o. For practical purposes, the fluctuations are ignored by averaging the K c of several days which may be enough for irrigation scheduling. To better understand the causes of the variability in the values of the daily ratio K T /fIRd shown in Fig. 7, we studied pairs of consecutive days with high and low K T /fIRd values and normalized them against their average value. The results shown in Fig. 10 indicate a negative correlation between K T /fIRd and ET o. It is likely that the increase in evaporative demand caused a decrease in almond tree canopy conductance (and thus a decrease in T), while it does not affect ET o. This is because the FAO Penman–Monteith equation (Allen et al. 1998) uses a hypothetic reference grass crop with a fixed value of canopy conductance that does not change with changes in evaporative demand. However, it is well known that stomatal aperture is sensitive to multiple environmental influences, and thus, canopy conductance will not be constant (Damour et al. 2010). On the other hand, the degree of coupling with the atmosphere of a tree crop is greater than that of a grass crop (Jarvis and McNaughton 1986), and thus, the canopy conductance of a tree crop will be more variable with changes in climatic conditions than that of a grass crop. Then, it is likely that the decrease in canopy conductance in almond trees on days of high evaporative demand had decreased K T relative to the lack of response of ET o.
References
Allen RG, Pereira LS (2009) Estimating crop coefficients from fraction of ground cover and height. Irrig Sci 28:17–34
Allen RG, Pereira LS, Raes D, Smith M (1998) Crop evapotranspiration: guidelines for computing crop water requirements. Irrigation and drainage. Paper no. 56. FAO, Rome
Auzmendi I, Mata M, del Campo J, Lopez G, Girona J, Marsal J (2011) Intercepted radiation by apple canopy can be used as a basis for irrigation scheduling. Agric Water Manag 98:886–892
Ayars JE, Johnson RS, Phene CJ, Trout TJ, Clark DA, Mead RM (2003) Water use by drip-irrigated late-season peaches. Irrig Sci 22:187–194
Bonachela S, Orgaz F, Villalobos FJ, Fereres E (1999) Measurement and simulation of evaporation from soil in olive orchards. Irrig Sci 18:205–211
Bonachela S, Orgaz F, Villalobos FJ, Fereres E (2001) Soil evaporation from drip-irrigated olive orchards. Irrig Sci 20:65–71
Campbell G, Norman J (1989) The description and measurement of plant canopy structure. In: Russel G, Marshall B, Jarvis PJ (eds) Plant canopies: their growth, form and function. Cambridge University Press, Cambridge, pp 1–19
Casadesus J, Mata M, Marsal J, Girona J (2011) Automated irrigation of apple trees based on measurements of light interception by the canopy. Biosyst Eng 108:220–226
Castel JR (1997) Evapotranspiration of a drip-irrigated clementine citrus tree in a weighing lysimeter. Acta Hortic 449:91–98
Consoli S, O’Connell N, Snyder R (2006) Estimation of evapotranspiration of different-sized navel-orange tree orchards using energy balance. Journal of irrigation and drainage engineering J Irrig Drain Eng ASCE 132:1–8
Damour G, Simonneau T, Cochard H, Urban L (2010) An overview of models of stomatal conductance at the leaf level. Plant, Cell Environ 33:1419–1438
Dekker S, Bouten W, Verstraten J (2000) Modelling forest transpiration from different perspectives. Hydrol Process 14:251–260
Doorenbos J, Pruitt WO (1977) Crop water requirements. Irrigation and drainage. Paper no. 24. FAO, Rome
Felipe AJ, Socias i Company R (1987) ‘Aylés’, ‘Guara’, and ‘Moncayo’ almonds. Hortic Sci 22:961–962
Fereres E, Martinich DA, Aldrich TM, Castel JR, Holzapfel CE, Schulbach H (1982) Drip irrigation saves money in young almond orchards. Calif Agric 36:12–13
Fereres E, Goldhamer DA, Sadras V (2012) Yield response to water of fruit trees and vines: guidelines. In: Steduto P, Hsiao TC, Fereres E, Raes D (eds) Cropyield response to water. Irrigation and drainage. Paper no. 66. FAO, Rome
Girona J (2006) La respuesta del cultivo del almendro al riego. Vida Rural 234:12–16
Girona J, del Campo J, Mata M, López G, Marsal J (2011) A comparative study of apple and pear tree water consumption measured with two weighing lysimeters. Irrig Sci 29:55–63
Goldhamer DA, Girona J (2012) Almond. In: Steduto P, Hsiao TC, Fereres E, Raes D (eds) Cropyield response to water. Irrigation and drainage. Paper no. 66. FAO, Rome
Goodwin I, Whitfiel DM, Connor DJ (2006) Effects of tree size on water use of peach (Prunus persica L. Bastch). Irrig Sci 24:59–68
Green S, McNaughton K, Wünshche JN, Clothier B (2003a) Modeling light interception and transpiration of apple tree canopies. Agron J 95:1380–1387
Green S, Clothier B, Jardine B (2003b) Theory and practical application of heat pulse to measure sap flow. Agron J 95:1371–1379
Jarvis PG, McNaughton KG (1986) Stomatal control of transpiration: scaling up from leaf to region. Adv Ecol Res 15:1–49
Lang ARG (1987) Simplified estimate of leaf area index from transmittance of the sun’s beam. Agric For Meteorol 41:179–186
Lenton R (2014) Irrigation in the twenty-first century: reflections on science, policy and society. Irrig Drain 63:154–157
López-Bernal A, Alcántara E, Testi L, Villalobos FJ (2010) Spatial sap flow and xylem anatomical characteristics in olive trees under different irrigation regimes. Tree Physiol 30:1536–1544
Lorite IJ, Santos C, Testi L, Fereres E (2012) Design and construction of a large weighing lysimeter in an almond orchard. Span J Agric Res 10:238–250
Mariscal MJ, Orgaz F, Villalobos FJ (2000) Modelling and measurement of radiation interception by olive canopies. Agric For Meteorol 100:183–197
Marsal J, Girona J, Casadesus J, López G, Stöckle CO (2013) Crop coefficient (K c) for apple: comparison between measurements by a weighing lysimeter and prediction by CropSyst. Irrig Sci 31:455–463
Marsal J, Johnson S, Casadesus J, López G, Girona J, Stöcke C (2014) Fraction of canopy intercepted radiation relates differently with crop coefficient depending on the season and the fruit tree species. Agric For Meteorol 184:1–11
Orgaz F, Villalobos FJ, Testi L, Fereres E (2007) A model of daily mean canopy conductance for calculating transpiration of olive canopies. Funct Plant Biol 32:178–188
Pereira AR, Green SR, Villa Nova NA (2007) Sap flow, leaf area, net radiation and the Priestley–Taylor formula for irrigated orchards and isolated trees. Agric Water Manag 92:48–52
Ross J (1981) The radiation regime and architecture of plants stands. Kluwer Academic Publisher, Dordrecth
Sanden B (2007) Fall Irrigation Management in a drought year for almonds, pistachios and citrus. Kern soil and water newsletter, September 2007. University of California Cooperative Extension, Kern County. http://cekern.ucdavis.edu/files/64007.pdf
Sanden B, Brown P, Snyder R (2012) New insights on water management in Almonds. In: 2012 Conference Proceedings. American Society of Agronomy. California Chapter: pp 88–91
Stevens RM, Ewenz CM, Grigson G, Conner SM (2012) Water use by an irrigated almond orchard. Irrig Sci 30:189–200
Stocker TF, Qin D, Plattner GK, Alexander LV, Allen SK, Bindoff NL, Bréon FM, Church JA, Cubasch U, Emori S, Forster P, Friedlingstein P, Gillett N, Gregory JM, Hartmann DL, Jansen E, Kirtman B, Knutti R, Krishna Kumar K, Lemke P, Marotzke J, Masson-Delmotte V, Meehl GA, Mokhov II, Piao S, Ramaswamy V, Randall D, Rhein M, Rojas M, Sabine C, Shindell D, Talley LD, Vaughan DG, Xie SP (2013) Technical Summary. In: Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds) Climate Change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp 33–115
Stöckle CO, Donatelli M, Nelson R (2003) CropSyst, a cropping systems simulation model. Eur J Agron 18:289–307
Testi L, Villalobos FJ (2009) New approach for measuring low sap velocities in trees. Agric For Meteorol 149:730–734
Villalobos FJ, Testi L, Moreno-Pérez MF (2009) Evaporation and canopy conductance of citrus orchards. Agric Water Manag 96:565–573
Villalobos FJ, Testi L, Orgaz F, García-Tejera O, López-Bernal A, González-Dugo MA, Ballester-Lurbe C, Castel JR, Alarcón-Cabañero JJ, Nicolás-Nicolás E, Girona J, Marsal J, Fereres E (2013) Modelling canopy conductance and transpiration of fruit trees in Mediterranean areas: a simplified approach. Agric For Meteorol 171–172:93–103
Acknowledgments
M Espadafor acknowledges the guidance provided by Prof. E. Fereres during the course of this study. M Espadafor is a recipient of research fellowship BES-2010-033883 from the Spanish Ministry of Science and Innovation. Financial support from the Spanish Ministry of Science and Innovation (AGL2009-07350) is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Marsal.
Rights and permissions
About this article
Cite this article
Espadafor, M., Orgaz, F., Testi, L. et al. Transpiration of young almond trees in relation to intercepted radiation. Irrig Sci 33, 265–275 (2015). https://doi.org/10.1007/s00271-015-0464-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00271-015-0464-6