Abstract
Purposes
The primary objective of this study (STEP trial) was to compare the efficacy of the polymer-based FemoSeal® vascular closure device (VCD) and the suture-based ProGlide® VCD in achieving hemostasis at the femoral access site after lower-limb arterial endovascular revascularization.
Materials and Methods
STEP was a multicenter randomized clinical trial including patients undergoing lower-limb arterial endovascular revascularization. The primary endpoint was technical success 5 h after the VCD intervention, defined as achievement of hemostasis without the need for a follow-up intervention at the access site and without a 2-g/dL drop in hemoglobin.
Results
Between December 2017 and April 2019, 113 patients were assigned to the FemoSeal® group (FS) and 117 to the ProGlide® group (PG). VCD interventions were technically successful for 90 FS patients (80%) and 58 PG patients (50%) (odds ratio, 3.98; 95% CI, 2.22 to 7.14; p < 0.0001). This difference in success rates between FS and PG is partly explained by more frequent recourse to manual compression (FS: n = 19; PG: n = 45) and an additional VCD (FS: n = 0; PG: n = 23) in the latter group. After 5 h, 87% of FS patients and 69% of PG patients resumed ambulation (odds ratio: 3.07; 95% CI: 1.93 to 6.15; p = 0.0016).
Conclusions
In patients undergoing lower-limb arterial endovascular revascularization, FemoSeal® was superior to ProGlide® in terms of technical success.
Clinical Trial Registration
Step trial was registered on http://ClinicalTrials.gov (NCT03192033).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last 20 years, endovascular therapy has become the standard of care in revascularization for vascular disease. Despite the development of radial artery access for coronary endovascular procedures, common femoral artery access is still the leading approach for a substantial proportion of peripheral interventions. [1,2,3] Because perioperative complications predominantly concern common femoral artery access, many efforts have been made to decrease sheath diameter and develop vascular closure devices (VCDs). [4] VCDs are used to close the incision made for percutaneous access and ensure hemostasis. Though many studies have shown advantages of VCD in terms of time to hemostasis, length of bed rest, early resumption of ambulation, and patients’ comfort and satisfaction, it has not been clearly demonstrated that VCD is safer than MC. [5].
For sheaths ≤ 8F, two groups of VCDs are described according to their mechanism of action: VCDs that deliver a plug and VCDs that physically close the arteriotomy site with use of either a nitinol clip or a suture. [5] So far, no recommendations for VCD selection criteria between both groups exist. However, recent publications about the arterial calcifications at the common femoral level could modify the VCDs choice. Indeed, osteoid metaplasia, a mature bone structure, is present in the majority of de novo common femoral artery lesions in patient with lower extremity arterial disease (LEAD). [6, 7] The presence of this solid and rigid calcified structure in the common femoral artery could jeopardize the successful deployment of VCDs that use of a needle and a suture which is not able to go through the calcifications. Moreover, some VCDs differ in term of secondary hemostasis. For Proglide® VCD (a suture-based vascular closure devices, Abbott Laboratories, Abbott Park, Ill, USA), the guidewire is left in place, while it is retrieved before implantation in the case of Femoseal® (a polymer disc, Terumo Corp., Tokyo, Japan). Consequently, no additional VCD can be deployed in the event of a FemoSeal® failure.
In the STEP trial, we aimed to compare Femoseal®, a collagen plug VCD, versus Proglide®, a suture-based VCD, in patients undergoing LEAD endovascular revascularization.
Materials and Methods
Study Design
The STEP trial (Etude prospective randommisée comparant l’efficacité des Systems de fermeTure artériElle Femoseal et Proglide après traitement endovasculaire de l’artérioPathie oblitérante des membres inférieurs) was a French national prospective randomized clinical trial conducted at seven centers and including 230 patients who went through diagnostic or therapeutic endovascular procedures for lower-limb disease. These patients were randomly assigned to one of two intervention arms, the FemoSeal® group (FS) or the ProGlide® group (PG). The trial was conducted in accordance with the ICH-E6, French Good Clinical Practice guidelines, and relevant regulations, and with ethical committee approval (#AU 1327, CPP Sud-Est IV, Clermont-Ferrand, France). Patients gave informed consent. The CONSORT statement has been used to report the trial's design, conduct, analysis and interpretation, and to assess the validity of its results. [8] The trial was designed by the first author in collaboration with our University Hospital Department of Clinical Research. This trial was registered on ClinicalTrials.gov (NCT03192033). STEP trial was funded by a grant from the French ministry of health (PHRC-IR 2017-A00894-49. Ref: RC16_0466).
Patients
Inclusion and exclusion criteria are provided in detail in Table 1. Briefly, patients were eligible for enrollment
if they had a history of lower-limb disease and if endovascular examination or treatment calling for a sheath of size 5F to 7F was indicated. Excluded were patients with a morbidity contraindicating same-day walking or a stent at the puncture site, or whose intervention involved a radial, brachial, or antegrade femoral puncture, or use of a sheath of size 8F or greater.
Randomization and Masking
Eligible patients were randomly assigned to FS and PG in a 1:1 ratio. Simple randomization was performed with the use of a web-based system and stratified by investigational site to insure proportional assignment. Due to the nature of the interventions, treating physicians were aware of the study arm assignments. Participating investigators were trained and certified in the use of both VCDs by the respective manufacturers before the center activation. Patients were blind to the type of VCD deployed until primary endpoint analysis. Site personnel who conducted any clinical follow-up assessments and individuals involved in data analysis were also blind to treatment assignment until primary endpoint analysis.
Treatments
Patients underwent conventional hospitalization. The type of anesthesia was left to the discretion of physicians. For each patient, a duplex scan-guided retrograde puncture of the common femoral artery was performed before randomization in order to determine the exact location of the access site. A 5F to 7F sheath was inserted, and 50 IU/kg of unfractionated heparin was administered intravenously. After diagnostic or therapeutic endovascular procedures, patients were randomly designated, intraoperatively, to receive FemoSeal®, or ProGlide® VCDs. The ProGlide® VCD involves a direct suture on either side of the arterial incision site, using a needle and polypropylene thread. The FemoSeal® VCD features a sandwich design: an intraluminal anchor and an extraluminal disk joined by a bioabsorbable suture. Both VCDs were deployed in accordance with the manufacturers’ instructions. When hemostasis was quickly attained, no compression dressing was used. Resumption of ambulation was assessed 5 h later by a walking test: patients were asked to walk down a hallway for 100 m and puncture site hemostasis was verified. Walking tests were conducted under medical and nurse supervision. Patients were discharged after overnight monitoring. Clinical examinations were scheduled for immediately, 5 h, 1 d, and 30 d after the procedures. All patients have duplex scans at 30 d. For both study arms, postoperative medical care included antiplatelet treatment. Initiation and discontinuation of antiplatelet medications were documented.
Outcomes
The primary endpoint was VCD technical success, which was assessed 5 h after the procedure and defined as hemostasis without the need for an additional VCD, MC, or another access site intervention, and without a 2-g/dL reduction in hemoglobin.
Potential major adverse events at 30 d accounted for by the study included death from any cause, stroke, myocardial infarction, and major amputation (transfemoral or transtibial). Stroke was defined as a sudden, focal neurological deficit of cerebrovascular origin, resulting in death or lasting longer than 24 h. Myocardial infarction was defined as showing presumed ischemic symptoms: chest pain, new ST-segment elevation of > 1 mm in ≥ 2 contiguous leads, and troponin levels > 2 times above normal. Access-related complications, assessed at 30 d, were categorized as minor or major. The latter included bleeding necessitating MC, an additional VCD, or another intervention; a decrease in hemoglobin decrease by 2 g/dL; or a pseudoaneurysm, local infection, acute ipsilateral leg ischemia, or nerve injury related to VCD deployment. The minor access site complication included access site hematoma or false aneurysm without the need of reintervention or a prolonged hospitalization. The procedure duration was measured from time of puncture to time of sheath removal. As mentioned previously, resumption of ambulation was assessed at 5 h. Clinical and hemodynamic improvements were assessed using the Rutherford classification and resting ankle-brachial index. Quality-of-life was assessed using the EuroQol EQ-5D-3L quality-of-life questionnaire, administered at the time of inclusion (baseline) and 30 d after the intervention. [9] Numbers of VCDs used were recorded and the total cost of VCDs per treated leg evaluated at the same purchase price.
Statistical Analysis
We calculated that a sample of 230 patients, randomly assigned in a 1:1 ratio, would be required to ensure 80% power for detection of a between-group difference of 12% in the technical success rate (FS: 97%; PG: 85%), at a two-sided alpha level of 0.05. Since no direct comparison exists between collagen and suture-based vascular closure devices for arterial closure following lower-limb arterial endovascular revascularization, a 12% difference in favor of Femoseal® was based on observational data (not published) and coronary studies. [10] Continuous data are presented as means plus or minus standard deviation or, for non-normal distributions or censored data sets, as medians with interquartile ranges. Categorical data are given as counts and percentages. Continuous data were compared by t-tests; categorical data, by chi-square tests. All patients were analyzed according to initial study arm assignment. The modified intention-to-treat population only included patients who had been randomized and met major inclusion criteria. Achievement of the primary endpoint was evaluated through an intention-to-treat analysis. For cases of bilateral femoral punctures, sensitivity analyses were performed and technical success was defined as technically successful VCD deployment on both sides. Randomization was stratified by center. Mixed models were used to analyze ABI change over time. All p-values are two-sided, p < 0.05 indicates a statistically significant difference, and no correction was made for multiple comparisons. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina).
Results
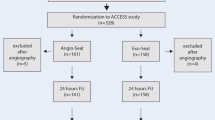
From December 2017 until April 2019, a total of 233 patients were enrolled in the trial and 230 underwent randomization (Fig. 1). Those 3 not randomized were withdrawn from analysis, not treated, and not followed up. Of the 113 FS patients, 109 underwent interventions; of the 117 PG patients, 109 did. Thus, 12 patients did not undergo interventions: 11 were ineligible and 1 was randomized twice. Follow-up at 30 d was possible for 106 FS and 107 PG patients. Of the remaining 5 patients, 1 had died and 4 were lost to follow-up by site investigators.
Baseline clinical and demographic characteristics of patients in the modified intention-to-treat population were well balanced between study arms (Table 2). Most patients exhibited intermittent claudication of the lower limbs justifying the treatment (FS: 86%; PG: 85%; p = 0.83) and had an American Society of Anesthesiologists (ASA) score of 2 or 3 (FS: 94%; PG: 88%; p = 0.49). There was no difference between arms in terms of antithrombotic medication: intravenous aspirin bolus (FS: 19%; PG: 18%, p = 0.86) or intravenous heparin bolus (FS: 95%; PG: 99%; p = 0.55).
Table 3 presents baseline angiographic and procedural data. The most frequently used sheath size in both arms was 6F. Frequencies of intervention types, intervention durations, and lesion types did not differ between arms. In the FS arm, we noted 11 patients with bilateral femoral access sites and 98 patients with unilateral femoral access sites (total: 120 femoral access sites). In the PG arm, we noted 19 patients with bilateral femoral access sites, 90 patients with unilateral femoral access sites (total 128 femoral access sites). VCDs were conventionally deployed to achieve hemostasis at a total of 248 femoral access sites (FS: 120; PG: 128) following lower-limb arterial endovascular revascularization. The total number of VCDs deployed was higher in PG (155) than in FS (118). At least one additional VCD was needed for 27 access sites (22%) in PG, but for none in FS (p < 0.0001). Among these 27 access sites, 8 ProGlide® and 23 FemoSeal® were used.
In the intention-to-treat analysis, VCD deployment was technically successful in 90 FS (80%) and 58 PG (50%) patients (odds ratio: 3.99; 95% CI: 2.22 to 7.14; p < 0.0001) (Fig. 2). Results were similar after adjustment for centers (data not shown). A sensitivity analysis was performed by excluding patients with manual compression for hemostasis and confirmed the superiority of Femoseal in term of technical success. (FemoSeal patients: 88.99% versus ProGlide patients: 62.39%) (p < 0.0001). In the PG, the use of a second VCD increases the technical success to 90% (98 patients). Table 4 showed the outcomes of primary and secondary ProGlide® deployment. A univariate analysis for failure analysis was performed and no factor was associated with a potential risk of failure (Table 5). The difference in technical success rates between study arms reflects recourse to additional VCDs (FS: 0 patients; PG: 23 patients) and greater need for MC (FS: 19 patients; PG: 45 patients) in the PG group. In a few cases, duplex-guided compression was needed to achieve hemostasis at femoral access sites (FS: 2; PG: 1). No patients experienced a 2-g/dL drop in hemoglobin.
For FS and PG, respectively, mean procedure durations were 65 ± 32 and 68 ± 32 min (p = 0.46); lengths of postoperative bedrest, 349 ± 78 min and 329 ± 61 min (p = 0.06); and hospital stays, 2.7 ± 3.8 d and 2.4 ± 3.1 d (p = 0.79); while 5 h after the procedure, 87% and 69% of patients could walk 100 m (odds ratio: 3.07; 95% CI: 1.53 to 6.15; p = 0.0016) and MC was needed in 4% and 1% of cases (p = 0.36).
At 30 d (see Table 6), 5 complications were noted. Complications in FS included 1 pseudoaneurysm treated with MC, 1 ipsilateral iliac artery rupture with retroperitoneal bleeding after balloon angioplasty and treated with a covered stent, 1 hematoma that required reintervention for evacuation and 1 procedure aborted due to patient agitation; and in PG, 1 contralateral femoral bifurcation thrombosis treated with thrombectomy and femoro-popliteal bypass. In all, there were 1 death and 16 rehospitalizations. The rate of major complications related to access sites differed (FS: 20%; PG: 56%; odds ratio: 0.20; 95% CI: 0.11 to 0.37; p < 0.0001). For both FS and PG, Rutherford classification and resting ABI had significantly improved at 30 d relative to baseline, there being no significant difference between the study arms.
EQ-5D-3L questionnaires were completed by 186 patients at 30 d. There were no major differences between arms for any of the EQ-5D-3L categories: mobility (p = 0.64), self-care (p = 0.48), usual activities (p = 0.47), pain (p = 0.31), or anxiety (p = 0.86).
In all, 118 FemoSeal® and 155 ProGlide® VCDs were used. As their unit prices are the same, the additional cost per treated leg in PG was 14%. The additional cost per patient varied between 23 and 30%, depending on the number of legs treated (FS mean: 1.09; PG mean: 1.16).
Discussion
The STEP trial was designed as a head-to-head comparison of a VCD using an intraluminal anchor (FemoSeal®) with a direct-suture VCD (ProGlide®) in lower-limb arterial endovascular revascularization patients. Five hours after the procedure, FemoSeal® was associated with a higher rate of technical success. Interestingly, we also noted that resumption of ambulation was significantly shorter in FS and that perioperative clinical outcomes were similar between study arms.
The primary endpoint, VCD technical success, was considered to be attained if hemostasis was effective, there was no need for another access site intervention, and hemoglobin levels did not fall by 2 g/dL. Poorer ProGlide® technical success reflects greater PG recourse to extra VCDs and MC. However, the STEP protocol did not allow to distinguish bleeding as a small bruising, an oozing, a pulsatile bleeding, or the cause of an enlarging hematoma, which could overestimate the failures in the PG arm. A sensitivity analysis was performed by excluding patients with manual compression for hemostasis and confirmed the superiority of Femoseal in term of technical success. (FemoSeal patients: 88.99% versus ProGlide patients: 62.39%) (p < 0.0001). This implies that the results of the primary analysis were not affected by procedures with manual compression for hemostasis. As safety endpoints, we considered both major and minor access site complications. We did not see any difference between groups in rates of local infections, acute ipsilateral leg ischemias, or nerve injuries. The higher frequency of technical failure in PG did not, furthermore, translate to longer hospitalizations. However, Wilde et al. suggest that VCD technical success may impact same-day discharge rates for ambulatory patients because it is a requisite for some interventionists. [11] Additionally, some physicians maintain that VCDs are not necessary for day-case peripheral vascular intervention as MC is a safe alternative. [12].
The presence of calcifications and osteoid metaplasia at the common femoral artery location, for example, can make needle pullback difficult when using the direct-suture ProGlide® VCD, even with ultrasound guidance. Herein, fluoroscopy was not used to assess the calcifications. And the intravascular anchor of the FemoSeal® VCD could cause arterial stenosis or occlusion, [13] or if incorrectly deployed, result in dissection, thrombosis, or plaque rupture. [14] While placement of the ProGlide® VCD might seem to require more technical skill, a systematic review of VCDs for femoral artery puncture sites did not suggest any difference between the FemoSeal® and ProGlide® in this regard. [5] It is also important to note that STEP trial procedures were performed by trained vascular interventionalists certified in the use of both devices by their respective manufacturers. Moreover, we did not observe any difference in trends across the study centers. Costs were higher in PG. The extra cost per patient ranged between 23 and 30%. Two factors may explain this difference: technical failure, of course, but also device characteristics. For ProGlide® VCDs, the guidewire is left in place, while it is retrieved before implantation in the case of FemoSeal®. Consequently, no additional VCD can be deployed in the event of a FemoSeal® failure. Thus, in our study, the FS failure rate was 17%, but no additional VCDs were used in such cases. However, the VCD cost estimates rely on French centers that may differ from other countries.
There have been few head-to-head comparisons of suture-mediated and polymer-based VCDs: most compare devices to MC. [5] A distinction should be made between intravascular and extravascular polymer-based VCDs. The former employ an intraluminal anchor, while the latter deploy a plug in the extravascular space adjacent to the puncture site. Recent randomized trials suggest extravascular polymer-based VCDs are associated with higher failure rates necessitating MC than intravascular polymer-based VCDs. [15, 16] In the latter category, FemoSeal® may seem simpler to use than AngioSeal® (Terumo Corp., Tokyo, Japan) as anchor deployment for FemoSeal® is mostly automatic. However, FemoSeal® is not available in the USA and has not been evaluated as often.
In a Cochrane review, Robertson et al. [17] compared the efficacy and safety of polymer-based and suture-based VCDs in achieving hemostasis for percutaneous arterial puncture of the common femoral artery, identifying 5 randomized trials. [12, 18,19,20,21] The polymer-based VCDs concerned were of intravascular (4 studies) or extravascular (1 study) design. ProGlide® was employed in four of the studies. Most trials included patients undergoing percutaneous coronary procedures or diagnostic catheterization. Robertson et al. showed polymer-based VCDs were associated with significantly greater technical success. No difference was noted in terms of frequency of pseudoaneurysms or retroperitoneal hemorrhages. In a recent randomized trial by Kara et al. [10], complications and device failure rates were lower for AngioSeal® than for ProGlide®. All these findings indicate differences in efficacy between VCDs and support the use of intravascular polymer-based devices, as do our own findings.
In the STEP trial, perioperative clinical outcomes were similar between study arms, and no access site infections were reported. Though Robertson et al. found such infections to be more common for VCDs than for MC, they reported similar infections rates between polymer-based and suture-based devices.
Study Limitations
Generalization of STEP outcomes is limited by the study’s narrow inclusion and exclusion criteria. To illustrate, our findings cannot be extrapolated to antegrade access, even though there are data suggesting FemoSeal® safety and efficacy for this route. [22] Furthermore, brachial access was also excluded because we are aware of the high incidence of complications and additional access-related interventions with which it is associated. [23] Nor do the study findings apply to patients undergoing percutaneous coronary interventions. In such patients, we may expect femoral arteries to be less atheromatous, so that suture-mediated VCDs might indeed prove more effective. Finally, STEP trial physicians were not blind to treatment assignment, so evaluation bias cannot be excluded. Finally, STEP trial physicians were not blind to treatment assignment, so evaluation bias cannot be excluded, such as the need of manual compression or the use of a second VCD in the ProGlide® arm. For instance, the ProGlide® action could not be always immediate and may require a short manual compression. Moreover, since the guidewire is left with the ProGlide®, it could increase the use of a second VCD.
Conclusion
The STEP randomized trial showed that the rate of technical success was higher for the intravascular polymer-based FemoSeal® VCD than for the suture-mediated ProGlide® VCD in patients undergoing lower-limb arterial endovascular revascularization. These findings argue for greater use of FemoSeal® to ensure more effective peripheral vascular interventions, especially among outpatients.
Abbreviations
- FS:
-
= FemoSeal study arm
- MC:
-
Manual Compression
- PG:
-
ProGlide study arm
- VCD:
-
Vascular Closure Device
References
Hvelplund A, Galatius S, Madsen M, Rasmussen JN, Sorensen R, Fosbol EL, et al. Influence of distance from home to invasive centre on invasive treatment after acute coronary syndrome: a nationwide study of 24 910 patients. Heart. 2011;97(1):27–32. https://doi.org/10.1136/hrt.2010.203901.
Steffenino G, Dellavalle A, Ribichini F, Russo P, Conte L, Dutto S, et al. Ambulation three hours after elective cardiac catheterisation through the femoral artery. Heart. 1996;75(5):477–80. https://doi.org/10.1136/hrt.75.5.477.
Ward SR, Casale P, Raymond R, Kussmaul WG 3rd, Simpfendorfer C. Efficacy and safety of a hemostatic puncture closure device with early ambulation after coronary angiography. Angio-Seal Invest Am J Cardiol. 1998;81(5):569–72. https://doi.org/10.1016/s0002-9149(97)00970-3.
Burns P, Gough S, Bradbury AW. Management of peripheral arterial disease in primary care. BMJ. 2003;326(7389):584–8. https://doi.org/10.1136/bmj.326.7389.584.
Noori VJ, Eldrup-Jorgensen J. A systematic review of vascular closure devices for femoral artery puncture sites. J Vasc Surg. 2018;68(3):887–99. https://doi.org/10.1016/j.jvs.2018.05.019.
Davaine JM, Quillard T, Chatelais M, Guilbaud F, Brion R, Guyomarch B, et al. Bone like arterial calcification in femoral atherosclerotic lesions: prevalence and role of osteoprotegerin and pericytes. Eur J Vasc Endovasc Surg. 2015. https://doi.org/10.1016/j.ejvs.2015.10.004.
Herisson F, Heymann MF, Chetiveaux M, Charrier C, Battaglia S, Pilet P, et al. Carotid and femoral atherosclerotic plaques show different morphology. Atherosclerosis. 2011;216(2):348–54. https://doi.org/10.1016/j.atherosclerosis.2011.02.004.
Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340: c869. https://doi.org/10.1136/bmj.c869.
EuroQol G. EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. https://doi.org/10.1016/0168-8510(90)90421-9.
Kara K, Mahabadi AA, Rothe H, Muller P, Kruger J, Neubauer H, et al. Safety and effectiveness of a novel vascular closure device: a prospective study of the ExoSeal compared to the Angio-Seal and ProGlide. J Endovasc Ther. 2014;21(6):822–8. https://doi.org/10.1583/14-4744MR.1.
Wilde NT, Bungay P, Johnson L, Asquith J, Butterfield JS, Ashleigh RJ. Outpatient angioplasty and stenting facilitated by percutaneous arterial suture closure devices. Clin Radiol. 2006;61(12):1035–40. https://doi.org/10.1016/j.crad.2006.07.008.
Kasthuri R, Karunaratne D, Andrew H, Sumner J, Chalmers N. Day-case peripheral angioplasty using nurse-led admission, discharge, and follow-up procedures: arterial closure devices are not necessary. Clin Radiol. 2007;62(12):1202–5. https://doi.org/10.1016/j.crad.2007.05.016.
Kirchhof C, Schickel S, Schmidt-Lucke C, Schmidt-Lucke JA. Local vascular complications after use of the hemostatic puncture closure device Angio-Seal. Vasa. 2002;31(2):101–6. https://doi.org/10.1024/0301-1526.31.2.101.
Sharma R, Vamanan K, Gupta K. Treatment of Angio-Seal(R) vascular closure device-induced acute femoral artery occlusion with SilverHawk(R) directional atherectomy. Cureus. 2016;8(12): e910. https://doi.org/10.7759/cureus.910.
Ketterle J, Rittger H, Helmig I, Klinghammer L, Zimmermann S, Hohenforst-Schmidt W, et al. Comparison of Exo-Seal((R)) and Angio-Seal ((R)) for arterial puncture site closure: a randomized, multicenter, single-blind trial. Herz. 2015;40(5):809–16. https://doi.org/10.1007/s00059-015-4306-3.
Schulz-Schupke S, Helde S, Gewalt S, Ibrahim T, Linhardt M, Haas K, et al. Comparison of vascular closure devices vs manual compression after femoral artery puncture: the ISAR-CLOSURE randomized clinical trial. JAMA. 2014;312(19):1981–7. https://doi.org/10.1001/jama.2014.15305.
Robertson L, Andras A, Colgan F, Jackson R. Vascular closure devices for femoral arterial puncture site haemostasis. Cochrane Database Syst Rev. 2016;3:CD09541. https://doi.org/10.1002/14651858.CD009541.pub2.
Hattab M. A randomized trial comparing two vascular closure devices: PROGLIDE and the novel EXOSEAL aIer percutaneous femoral procedures. J Am Coll Cardiol. 2012;60(17):B112.
Martin JL, Pratsos A, Magargee E, Mayhew K, Pensyl C, Nunn M, et al. A randomized trial comparing compression, Perclose Proglide and Angio-Seal VIP for arterial closure following percutaneous coronary intervention: the CAP trial. Catheter Cardiovasc Interv. 2008;71(1):1–5. https://doi.org/10.1002/ccd.21333.
Park Y, Roh HG, Choo SW, Lee SH, Shin SW, Do YS, et al. Prospective comparison of collagen plug (Angio-Seal) and suture-mediated (the Closer S) closure devices at femoral access sites. Korean J Radiol. 2005;6(4):248–55. https://doi.org/10.3348/kjr.2005.6.4.248.
Jensen J, Saleh N, Jensen U, Svane B, Jonsson A, Tornvall P. The inflammatory response to femoral arterial closure devices: a randomized comparison among FemoStop, AngioSeal, and Perclose. Cardiovasc Intervent Radiol. 2008;31(4):751–5. https://doi.org/10.1007/s00270-008-9323-7.
Tagliaferro FB, Orgera G, Mascagni L, Laurino F, Tipaldi MA, Cariati M, et al. FemoSeal((R)) vascular closure device for antegrade common femoral artery access: Safety and technical notes. J Vasc Access. 2020;21(1):79–85. https://doi.org/10.1177/1129729819854593.
Meertens MM, de Haan MW, Schurink GWH, Mees BME. A stopped pilot study of the ProGlide closure device after transbrachial endovascular interventions. J Endovasc Ther. 2019;26(5):727–31. https://doi.org/10.1177/1526602819862775.
Acknowledgments
We would like to thank clinical research assistants Patrice Chauveau, Sandrine Renaud, and Julie Jaulin for their excellent technical support in monitoring data. Language editing of the first version of this manuscript was provided by Jason Miller of SQUID Translation, funded by the Center Hospitalier Universitaire de Nantes (Nantes teaching hospital).
Funding
This study was funded by a grant from the French Ministry of Health (PHRC-IR 2017-A00894-49; ref: RC16_0466). The funder of the study had no role in study design; data collection, analysis, or interpretation; or drafting of this report.
Author information
Authors and Affiliations
Contributions
Dr. Gouëffic reports research funding from Bard, Medtronic, Terumo, and W.L. Gore; and personal fees and grants from Abbott, Bard, Biotronik, Boston Scientic, Medtronic, Terumo, Vygon, and W.L. Gore (modest). Dr. Schneider reports personal fees from W.L. Gore (modest). Dr. Kaladji reports personal fees from W.L. Gore (modest). Dr. Nasr reports personal fees from Medtronic, Biotronik, and W.L. Gore (modest). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Corresponding author
Ethics declarations
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study. Consent for publication was obtained for every individual person’s data included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gouëffic, Y., Picquet, J., Schneider, F. et al. A Randomized Trial Comparing Polymer Versus Suture-Based Vascular Closure Devices for Arterial Closure Following Lower-Limb Arterial Endovascular Revascularization. Cardiovasc Intervent Radiol 44, 1883–1892 (2021). https://doi.org/10.1007/s00270-021-02940-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-021-02940-z