Abstract
Background and Purpose
Data on the management of large vessel occlusion in patients with anterior circulation acute ischemic stroke (AIS) due to underlying intracranial stenosis are scarce. The aim of this retrospective study was to compare endovascular treatment and outcome in AIS patients with and without underlying stenosis of the M1 segment.
Materials and Methods
A total of 533 acute stroke patients with an isolated M1 occlusion who underwent mechanical thrombectomy between 02/2010 and 08/2017 were included. Underlying intracranial atherosclerotic stenosis (ICAS) was present in 10 patients (1.9%), whereas 523 patients (98.1%) had an embolic occlusion without stenosis.
Results
There was no difference in age, admission National Institutes of Health Stroke Scale, risk factors, Alberta stroke program early CT score or collaterals between the groups. Procedure time (155 vs 40 min, P = 0.001) was significantly longer in the ICAS group where rescue stent-angioplasty was performed in all patients. There was no statistical difference in final modified thrombolysis in cerebral infarction score between both groups (70 vs 88%, P = 0.115). Favorable outcome (modified Rankin Scale ≤ 2) at 90 days was less frequent in patients with ICAS than in the embolic group (0 vs 49.4%, P = 0.004). The mortality rate tended to be higher in the ICAS group (44.4 vs 19.4%, P = 0.082).
Conclusion
In patients with AIS, rescue therapy with stent placement to treat underlying ICAS of the M1 segment is technically feasible; however, in our study, a significantly lower rate of favorable outcome was observed in these patients compared to those with thromboembolic M1 occlusions.
Level of Evidence
Level 3, non-randomized controlled study.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The treatment of stroke patients with large vessel occlusion of the anterior circulation has dramatically changed following seven randomized controlled trials that showed endovascular thrombectomy to be superior to best medical treatment with intravenous plasminogen activator (IV rtPA) [1,2,3,4,5,6,7]. However, in some patients undergoing endovascular treatment (EVT), stenosis of the target vessel may become apparent after thrombectomy, suggesting underlying intracranial atherosclerotic stenosis (ICAS). This has implications for further management, as instant re-occlusion after successful recanalization is more frequent in patients with underlying ICAS (65 vs 3.3%) [8] and often requires rescue therapy. Data on the appropriate management of acute stroke patients with underlying ICAS of the anterior circulation are scarce, and no general consensus exists. The prevalence of ICAS varies among ethnic groups, being more common in Asians and African-Americans than in Caucasians [9, 10]. There is some discrepancy in the literature regarding estimates of its prevalence in Caucasians, which range from 1% [9] to 24% [11].
The aim of this study was to compare acute ischemic stroke (AIS) patients with an occlusion of the M1 segment due to an underlying stenosis (group 1) and patients with embolic M1 occlusions without underlying stenosis (group 2) undergoing endovascular recanalization in a large-volume center in Europe. Clinical and demographic aspects, recanalization rates, periprocedural complication rates, intracranial hemorrhage, and clinical outcome were compared between patients with ICAS-related and those with embolic occlusions.
Methods
Patients
All consecutive stroke patients who underwent stent-retriever thrombectomy at a tertiary university hospital center between 02/2010 and 08/2017 who met the inclusion criteria were retrospectively reviewed. The criteria were: (1) isolated M1 occlusion, (2) acute symptom onset with a neurologic deficit corresponding to the occluded middle cerebral artery (MCA) territory, (3) National Institutes of Health Stroke Scale (NIHSS) ≥ 4 (except for isolated aphasia or hemianopia), (4) time from symptom onset to groin puncture < 6 h, if presentation was later, a perfusion study demonstrating relevant cerebral blood flow/cerebral blood volume mismatch indicating penumbra and salvageable tissue, (5) stent-retriever thrombectomy as the primary method. The exclusion criteria were: (1) multiple vessel occlusions or tandem occlusions, (2) another stroke etiology such as Moya–Moya, dissection, or vasculitis, (3) unsuccessful reperfusion, corresponding to modified thrombolysis in cerebral infarction (mTICI score 0) since no distinction between ICAS and an embolic occlusion could be made, (4) use of angioplasty as a rescue procedure to compress a thrombus resistant to thrombectomy.
Patients were divided into two groups: group 1 comprised those with an underlying ICAS and group 2, patients with an embolic occlusion without underlying stenosis. An ICAS was defined as a fixed focal stenosis > 70% of the vessel segment subjected to thrombectomy, not sensitive to vasoactive substances (nimodipine), with impairment of perfusion of the dependent territory, or a tendency to early re-occlusion. The study was performed in accordance with the applicable ethical guidelines and with the permission of the institutional review board.
On admission, initial clinical evaluation was conducted by a stroke neurologist in the emergency department. Noninvasive magnetic resonance imaging (MRI) was performed, which included the following sequences: axial diffusion-weighted imaging, axial fluid-attenuated inversion recovery, susceptibility-weighted imaging, intracranial time-of-flight angiography, contrast-enhanced cervical angiography, perfusion, and post-gadolinium spin echo T1-weighted imaging or computed tomography (CT) including thin-slice non-contrast CT, angiography of the intracranial and supraaortic vessels and a perfusion study. All patients with proven occlusion of the M1 segment received IV rtPA, if they presented within < 4.5 h of symptom onset and had no contraindications, before undergoing endovascular therapy (EVT). Patients presenting > 4.5 h were directly subjected to EVT.
For evaluation, data on the selected patients were obtained from the Stroke Registry, which lists all patients admitted to our hospital with a diagnosis of stroke. The data registered included the NIHSS score at presentation, the Alberta stroke program early CT (ASPECT) score and MRI ASPECT score, time from symptom onset to groin puncture, procedure time, additional IV rtPA, and type of anesthesia. Collateral flow on angiography was graded on a three-point scale: grade 0, no collaterals visible in the ischemic territory; grade 1, slow collaterals to the periphery of the ischemic site with persistence of some of the defect; grade 2, collaterals with complete angiographic blood flow to the ischemic territory by retrograde perfusion. Periprocedural complications, stent thrombosis, symptomatic intracerebral hemorrhage, and modified Rankin Scale (mRS) at 3 months were also recorded.
Thrombectomy
Selective intra-arterial digital subtraction angiography (DSA) was performed via a transfemoral approach on a biplane (Axiom Artis zee; Siemens, Erlangen, Germany) or monoplane (Artis zee multipurpose; Siemens) high-resolution angiographic system using Iopamiro 300 (Iopamidol, Bracco, Switzerland) for vessel opacification. Depending on the clinical status of the patient, the intervention was performed under general anesthesia or conscious sedation. For safe access, an 8- or 9-French (Fr) sheath (Terumo, Terumo Medical, Tokyo, Japan) was placed in the common femoral artery. Cerebral angiography was performed injecting both carotids and one vertebral artery, to assess the occlusion site and collateral status. A guiding catheter was advanced into the internal carotid artery (ICA) to provide stable support during the intervention and flushed continuously with heparinized sodium chloride solution (5 IU/ml). Stent-retriever thrombectomy (Solitaire, Covidien, Irvine, California, USA) was the first-line technique. When using a balloon guiding catheter (BGC) (Merci BGC, Concentric Medical, Mountain View, CA, USA), the balloon was inflated for flow arrest and the stent retriever was pulled back under constant suction applied through the BGC with two 60-ml aspiration syringes. When using a standard guiding catheter (Merci), a distal access catheter (DAC) was advanced over the push-wire of the stent retriever into the occluded vessel until becoming occlusive at the proximal thrombus end. As rescue therapies, various stent retrievers or aspiration thrombectomy were used at the discretion of the neurointerventionalist. Successful reperfusion was defined as mTICI score of 2b–3.
In the case of recanalization of the arterial occlusive lesion and the presence of wall irregularities in the treated vessel greater than 50% of the original vessel lumen, 2 mg nimodipine was added to the pressurized flush bag connected to the guiding catheter for continuous irrigation. A control run was performed after 10 min to check for changes in vessel irregularities: in case of regression it was considered a vasospasm, when they persisted an underlying ICAS was suspected.
Intracranial Stenting
Persistent severe intracranial stenosis > 70% and a tendency toward early re-occlusion were considered to be due to underlying ICAS. For quantification of the stenosis, the Warfarin Aspirin Symptomatic Intracranial Disease criteria were used [12]. If re-occlusion occurred, the thrombectomy was repeated and 200–300 mg of aspirin was administered. A repeat angiographic run was performed after 10 min. When stenosis persisted, an exchange microwire (Transend 0.014, 300 cm, Boston Scientific, Natick, MA, USA) was navigated distal to the lesion and percutaneous transluminal angioplasty (PTA) was performed with a non-compliant balloon (Gateway, Stryker Inc., Fremont, California, USA). The balloon diameter was adjusted to approximately 80% of the normal vessel diameter, and the shortest length that would cover the entire stenotic segment was chosen to minimize potential damage to perforating lateral lenticulostriate arteries in adjacent unaffected segments [13, 14]. The balloon was slowly inflated to nominal pressure. After deflation, the balloon was withdrawn, the guidewire was left in place, and a control run was performed to rule out a vessel perforation. The next control run was performed 10 min post-PTA, and when stenosis persisted or re-occlusion occurred we opted for stent implantation (n = 9 Wingspan-Stryker, Kalamazoo, Michigan, USA; n = 2 Xience Xpedition-Abbott Vascular, Santa Clara, CA) (Fig. 1). In most patients, a post-PTA was performed after stent implantation. If thrombus formation occurred within the stent, a glycoprotein IIb/IIIa antagonist abciximab (ReoPro, Eli Lilly, Indianapolis, USA) was slowly injected intra-arterially via the guide catheter (5–10 mg). Follow-up angiography was performed 10–20 min after stent deployment and if no change or thrombus formation was observed, the procedure was terminated. Procedural and technical details are summarized in Table 2.
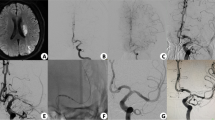
79-year-old male patient with fluctuating, left hemisyndrome, and acute worsening; NIHSS 13, MR ASPECT score of 9 (Patient No. 5). (A) DSA image in the ap projection after thrombectomy shows high-grade stenosis of the M1 segment. (B) Persistent stenosis after PTA (Gateway 2 × 20 mm) with early re-occlusion. (C) Post-dilatation after stent deployment (Wingspan 2.5 × 9 mm). (D) Final angiogram demonstrating a patent stent with slight residual narrowing. (E) Follow-up CTA on the following day demonstrating a patent intracranial stent. The patient died 5 days after stroke due to ST elevation infarct and respiratory decompensation (mRS-6)
Post-interventional Procedure
After EVT, all patients were admitted to the stroke unit and closely monitored. In accordance with our standard protocol, follow-up imaging was performed after 24 h with CT or MRI. Intracranial hemorrhage (ICH) was classified according to the European–Australasian Acute Stroke Study Classification (ECASS II) [15]. Symptomatic hemorrhage was defined as parenchymal hematoma causing mass effect, with clinical deterioration (increase in NIHSS score ≥ 4). Clinical outcome was prospectively assessed 3 months after the stroke using the modified Rankin Scale (mRS). All patients were invited for a 3-month outpatient visit and if they were unable to attend, mRS was assessed by a structured phone interview.
Statistical Analysis
Statistical analyses were performed using SPSS (version 21.0; IBM, Armonk, New York, USA). Data were tested for normality of distribution and equal standard deviations to determine whether parametric or nonparametric assumptions should be used. The baseline demographic characteristics, risk factors, imaging characteristics, and clinical outcomes were compared between the ICAS and embolic group. The baseline characteristics and clinical outcomes of the two groups were compared with the χ2 test for categorical and binary data, and the Mann–Whitney U test was used for continuous data. A P value less than 0.05 was considered to indicate a statistically significant difference.
Results
A total of 533 AIS patients with an isolated M1 occlusion who met the inclusion criteria were treated during the defined study period, 10 (1.9%) with an underlying ICAS (group 1), and 523 (98.1%) with an embolic occlusion without underlying stenosis (group 2). The baseline patient characteristics are shown in Table 1. There were no significant differences in stroke risk factors between the groups (i.e., hypertension, diabetes, coronary artery disease, hypercholesterinemia, smoking, previous stroke). The two groups did not show difference in NIHSS at admission, blood pressure at admission, international normalized ratio (INR), glucose, platelets, antithrombotics, ASPECT score, or collaterals. The time from symptom onset to admission (mean 190 vs 133 min, P = 0.287) and symptom onset to reperfusion (395 vs 277 min, P = 0.071) tended to be longer in ICAS patients, but did not reach statistical significance. Procedure time (155 vs 40 min, P < 0.001) was longer in ICAS patients. All patients in the ICAS group, except one patient with a periprocedural M1 rupture, received antiplatelet therapy during intervention (n = 3 aspirin only, n = 1 abciximab only, n = 1 aspirin + clopidogrel, n = 4 aspirin + abciximab) (Table 2). There was no statistically significant difference in final reperfusion success (as measured by mTICI) between groups (70% vs 88%, P = 0.115) or the rate of sICH (10 vs 3.1%, P = 0.255). Three stents (30.0%) were found to be occluded at follow-up (Fig. 2), seven stents (70.0%) were open. Favorable outcome (mRS ≤ 2) at 90 days was observed less frequently in patients with ICAS than in patients in the embolic group (0 vs 49.4%, P = 0.004). The mortality rate tended to be higher in the ICAS group (44.4 vs 19.4%, P = 0.082), but the difference did not reach statistical significance.
72-year-old male patient with acute left hemiparesis; NIHSS 9, MR ASPECT score of 4 (Patient No. 4). (A) DSA image in the ap projection after thrombectomy shows high-grade stenosis of the distal M1 segment. (B) DSA after stent deployment (Wingspan 2.5 × 9 mm) shows early re-occlusion at the proximal stent. (C) Deployment of a second stent using the telescoping technique (Xience Xpedition 3 × 8 mm). (D) Final angiogram demonstrating some contrast penetration with minimal perfusion of the MCA territory (mTICI 1). (E) Follow-up CTA on the following day demonstrating an occluded stent. Clinical outcome at 3 months was mRS of 4
Overall, the rate of periprocedural complications was low. One M1-rupture occurred in a patient in the ICAS group during stent deployment and was occluded with coils. In patients in the embolic group, a total of four perforations were reported; two ceased spontaneously and, in the other two, coiling was necessary. Peri-interventional ICA dissections occurred in 13 patients (2.5%) in the embolic group; four required stenting. In one patient in the embolic group, inadvertent stent-retriever detachment in the M1 segment occurred; however, this did not require any further treatment and remained open.
One patient (No. 10) with a left M1 occlusion initially treated with stent-retriever thrombectomy showed good reperfusion (mTICI 3) with subtle vessel irregularities (< 50%) on the final angiogram and was admitted to the stroke unit. In response to clinical deterioration 14 h after the initial procedure, a new DSA showed re-occlusion of the M1 segment. Thrombectomy was repeated, but due to a tendency toward re-occlusion a postponed stenting was performed.
Discussion
Rescue therapy with stent placement for underlying ICAS of the M1 segment in patients with AIS is technically feasible, shows high rates of good reperfusion, and appears to be technically safe. However, in our study population, a significantly lower rate of favorable outcome (mRS ≤ 2) at 90 days was observed in patients with ICAS compared to those with thromboembolic M1 occlusions.
Before large randomized stroke trials have provided evidence for mechanical thrombectomy in large vessel occlusion, PTA and stenting of intracranial arteries were used in patients with contraindications for IV rtPA or resistance to intra-arterial thrombolysis [16, 17]. Only few trials have addressed rescue treatment in patients with ICAS in acute stroke, most pooling data from anterior and posterior circulation leading to an inhomogeneous population, thus making interpretation difficult [18,19,20,21,22,23]. In the SAMMPRIS trial, aggressive medical management plus stenting failed to show an effect as compared to aggressive medical management alone in patients with a recent transient ischemic attack due to a high-grade intracranial artery stenosis (< 70%) [24]. However, patients with hyperacute stroke are in a different clinical situation and present with complete vessel occlusion due to local thrombus formation at the level of a vulnerable plaque. In this setting, rescue treatment with PTA and/or stenting is the only viable option to maintain vessel patency.
Our findings tend to reflect published data from centers across Europe and North America and a Korean study by Baek et al. on rescue treatment after failure of mechanical thrombectomy only including large artery occlusions of the anterior circulation, the last reporting a favorable outcome (mRS 0–2) in 35.3%, and mortality rates of 23.5% [25]. Our clinical results are in contrast to most studies from Asia which reported high rates of successful reperfusion (mTICI 2b–3) in their ICAS group, in some even exceeding the rates in the embolic group: Yoon et al. (95.0 vs 81.8%) [19], Jia et al. (95.7 vs 96.8%) [20], Lee et al. (100%, no embolic group) [18]. This translated to high favorable outcome rates (mRS 0–2) at 3 months as compared to the embolic group: Yoon et al. (62.5 vs 38.6%), Jia et al. (63.8 vs 51.6%), Lee et al. (66.7%, no embolic group). This discrepancy may be due to several factors. First, a different definition of ICAS was used across studies. In the study by Yoon et al., among 40 patients with ICAS, the authors included 11 patients with severe stenosis of the target artery on diagnostic angiography, without complete occlusion and thus not requiring thrombectomy [19]. This might have had a substantial impact on the patient outcome since a complete arrest of antegrade flow never occurred in these patients. In contrast, in our study, only patients with complete vessel occlusion and underlying ICAS, as revealed after failed recanalization by thrombectomy, were included. Second, the overall higher prevalence of ICAS in the Asian population may result in higher case load and greater experience of the treating physicians. The overall rate of underlying ICAS in our population (1.9%) is low, a finding that reflects regional differences and lower prevalence in Western populations [21]. Third, in our population, there was a tendency toward a longer time from symptom onset to admission in the ICAS group, although this was not statistically significant. Fluctuating clinical symptoms and better collaterals may have led to a later presentation of these patients.
Questions open to debate that may impact the overall patient outcome are the adequate antithrombotic regime and the stent choice. In the study by Forbrig et al. including acute stroke patients with underlying stenosis or dissection of the anterior or posterior circulation, functional independency after 3 months was reported in 37% of patients with a patent stent, as compared to 0% when stent occlusion occurred [23]. Thus, stent re-occlusion may be considered a major factor of poor clinical outcome. The authors concluded that double antiaggregation with aspirin and clopidogrel may not be sufficient in the acute setting, since in the patients with early stent occlusion or severe restenosis two-thirds had received this combination. Woo et al. demonstrated better angiographic outcomes and higher proportion of favorable mRS (0 –2) at 3 months, without increasing the rate of hemorrhagic transformation in patients receiving a glycoprotein IIb/IIIa inhibitor as compared with those who did not during permanent stent implantation as a rescue procedure in acute intracranial occlusion [29]. Balancing the risk of an intracranial hemorrhage and stent occlusion remains a challenge. Stent occlusion occurred in two patients in our study who have received 250 or 300 mg aspirin and 5.000 IU heparin only, due to hypodense demarcation of the basal ganglia, which has been shown to increase the risk of intracranial hemorrhage [26,27,28]. However, this may not be sufficient in the acute setting.
Concerning the stent choice, different stents have been used for rescue stent-angioplasty in acute stroke patients with an underlying stenosis and include Enterprise, Solitaire, Neuroform, Wingspan, or Acclino [23]. Woo et al. compared Solitaire AB with other stents for rescue treatment and demonstrated significantly shorter procedure translating into a favorable outcome [29]. The Wingspan stent was almost exclusively used in our study. It is one of the first stents that has been approved for intracranial use by the Food and Drug Administration in 2005, however may have some potential limitations which should be considered. First, an exchange maneuver is required, increasing the peri-interventional risk and prolonging the procedure time. Second, rate of restenosis up to 30% has been reported [30, 31].
The median procedure time for the ICAS group in our study, 155 min (129–170 min), was substantially longer than in several Asian studies and is more in line with the duration reported by Baek et al. (155.3 ± 64.0)[25]. When faced with a patient with ICAS, we take an escalating approach starting with the least invasive method before proceeding with angioplasty or stenting, which is overall more time-consuming. A vulnerable plaque at the stenotic vessel segment incites aggregation of platelets leading to local thrombus formation and eventually to vessel occlusion. Thrombectomy seems to effectively remove fresh clots, and partial recanalization is usually seen in ICAS patients. At the same time, a stent retriever may further disrupt the plaque, thus exposing thrombogenic material to the vessel lumen and promoting local thrombus formation and early re-occlusion [32]. To counteract this effect, we usually administer intravenous aspirin and repeat a control run after 10–20 min to look for any change. Only if severe stenosis persists or re-occlusion occurs do we advocate mechanical rescue therapy, primarily with PTA or eventually stent deployment. Both techniques (PTA and stent) have a potential risk of pushing plaque material into small perforating branches originating from the stenotic segment or in its immediate vicinity and causing new ischemia. This was reported in the SAMMPRIS trial; however, it is not of major importance in acute stroke patients because perforator stroke has usually already occurred due to vessel occlusion [24].
Our data suggest that Caucasian patients with an acute M1 occlusion and underlying ICAS have a significantly worse outcome than patients with embolic occlusions. However, rescue treatment with PTA and stenting is the only option in this scenario and should be considered. Since stent patency is one of the most important prognostic factors, optimizing the antithrombotic treatment is essential, which remains the matter of debate and is reflected by different antiplatelet/anticoagulation regimes in the literature. As shown in our study and by Forbrig, mono and double antiaggregation with aspirin and clopidogrel may not be sufficient in the acute setting and glycoprotein IIb/IIIa inhibitors should be considered. We acknowledge that our results need to be interpreted with caution because they were derived retrospectively from a single center and have the limitations inherent to this study type. In addition, due to regional differences in the prevalence of ICAS, the experience of centers in Asia is more extensive than in centers in Europe and North America.
Conclusion
In our experience, endovascular treatment of AIS patients with underlying ICAS is complex and time-consuming, albeit feasible. However, a significantly lower rate of favorable outcome (mRS ≤ 2) at 90 days was observed in these patients compared to those with thromboembolic M1 occlusions.
References
Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:1–11.
Campbell BCV, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–18.
Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–30.
Berkhemer OA, Fransen PSS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. New Engl J Med. 2015;372:11–20.
Saver JL, Goyal M, Bonafe A, Diener H-C, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285–95.
Bracard S, Ducrocq X, Mas JL, Soudant M, Oppenheim C, Moulin T, et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016;15:1138–47. https://doi.org/10.1016/S1474-4422(16)30177-6.
Mocco J, Zaidat OO, von Kummer R, Yoo AJ, Gupta R, Lopes D, et al. Aspiration thrombectomy after intravenous alteplase versus intravenous alteplase alone. Stroke. 2016;47:2331–8.
Kang D-H, Kim Y-W, Hwang Y-H, Park S-P, Kim Y-S, Baik SK. Instant reocclusion following mechanical thrombectomy of in situ thromboocclusion and the role of low-dose intra-arterial tirofiban. Cerebrovasc Dis. 2014;37:350–5.
Sacco RL, Kargman DE, Gu Q, Zamanillo MC. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study. Stroke. 1995;26:14–20.
Suri MFK, Qiao Y, Ma X, Guallar E, Zhou J, Zhang Y, et al. Prevalence of intracranial atherosclerotic stenosis using high-resolution magnetic resonance angiography in the general population: the atherosclerosis risk in communities study. Stroke. 2016;47:1187–93.
Wityk RJ, Lehman D, Klag M, Coresh J, Ahn H, Litt B. Race and sex differences in the distribution of cerebral atherosclerosis. Stroke. 1996;27:1974–80.
Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol. 2000;21:643–6.
Bose A, Hartmann M, Henkes H, Liu HM, Teng MMH, Szikora I, et al. A novel, self-expanding, nitinol stent in medically refractory intracranial atherosclerotic stenoses: the Wingspan study. Stroke. 2007;38:1531–7.
Fiorella D, Levy EI, Turk AS, Albuquerque FC, Niemann DB, Aagaard-Kienitz B, et al. US multicenter experience with the wingspan stent system for the treatment of intracranial atheromatous disease: periprocedural results. Stroke. 2007;38:881–7.
Hacke W, Kaste M, Fieschi C, Von Kummer R, Davalos A, Meier D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Lancet. 1998;352:1245–51.
Ueda T, Hatakeyama T, Kohno K, Kumon Y, Sakaki S. Endovascular treatment for acute thrombotic occlusion of the middle cerebral artery: local intra-arterial thrombolysis combined with percutaneous transluminal angioplasty. Neuroradiology. 1997;39:99–104.
Ramee SR. Catheter-based treatment for patients with acute ischemic stroke ineligible for intravenous thrombolysis. Stroke. 2004;35:e109–11.
Lee JS, Hong JM, Lee KS, Suh HI, Choi JW, Kim SY. Primary stent retrieval for acute intracranial large artery occlusion due to atherosclerotic disease. J Stroke. 2016;18:96–101.
Yoon W, Kim SK, Park MS, Kim BC, Kang HK. Endovascular treatment and the outcomes of atherosclerotic intracranial stenosis in patients with hyperacute stroke. Neurosurgery. 2015;76:680–6.
Jia B, Feng L, Liebeskind DS, Huo X, Gao F, Ma N, et al. Mechanical thrombectomy and rescue therapy for intracranial large artery occlusion with underlying atherosclerosis. J Neurointerv Surg. 2018;10:746–50.
Gascou G, Lobotesis K, Machi P, Maldonado I, Vendrell JF, Riquelme C, et al. Stent retrievers in acute ischemic stroke: complications and failures during the perioperative period. Am J Neuroradiol. 2014;35:734–40.
Al Kasab S, Almadidy Z, Spiotta AM, Turk AS, Chaudry MI, Hungerford JP, et al. Endovascular treatment for AIS with underlying ICAD. J Neurointerv Surg. 2017;9:948–51.
Forbrig R, Lockau H, Flottmann F, Boeckh-Behrens T, Kabbasch C, Patzig M, et al. Intracranial rescue stent angioplasty after stent-retriever thrombectomy: multicenter experience. Clin Neuroradiol. 2018. https://doi.org/10.1007/s00062-018-0690-4
Derdeyn CP, Chimowitz MI, Lynn MJ, Fiorella D, Turan TN, Janis LS, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet. 2014;383:333–41.
Baek JH, Kim BM, Kim DJ, Heo JH, Nam HS, Yoo J. Stenting as a rescue treatment after failure of mechanical thrombectomy for anterior circulation large artery occlusion. Stroke. 2016;47:2360–3.
Kaesmacher J, Kaesmacher M, Maegerlein C, Zimmer C, Gersing AS, Wunderlich S, et al. Hemorrhagic transformations after thrombectomy: risk factors and clinical relevance. Cerebrovasc Dis. 2017;43:294–304.
Loh Y, Towfighi A, Liebeskind DS, MacArthur DL, Vespa P, Gonzalez NR, et al. Basal ganglionic infarction before mechanical thrombectomy predicts poor outcome. Stroke. 2009;40:3315–20.
Loh Y, Liebeskind DS, Towfighi A, Vespa P, Starkman S, Saver JL, et al. Preprocedural basal ganglionic infarction increases the risk of hemorrhagic transformation but not worse outcome following successful recanalization of acute middle cerebral artery occlusions. World Neurosurg. 2010;74:636–40. https://doi.org/10.1016/j.wneu.2010.06.021.
Woo HG, Sunwoo L, Jung C, Kim BJ, Han MK, Bae HJ, et al. Feasibility of permanent stenting with solitaire FR as a rescue treatment for the reperfusion of acute intracranial artery occlusion. Am J Neuroradiol. 2018;39:331–6.
Wolfe TJ, Fitzsimmons BF. Long term clinical and angiographic outcomes with the Wingspan stent for treatment of symptomatic 50–99% intracranial atherosclerosis : single center experience in 51 cases. J Neurointerv Surg. 2009;1:40–3.
Henkes H, Miloslavski E, Lowens S, Reinartz J, Liebig T, Kühne D. Treatment of intracranial atherosclerotic stenoses with balloon dilatation and self-expanding stent deployment (WingSpan). Neuroradiology. 2005;47:222–8.
Mosimann PJ, Kaesmacher J, Gautschi D, Bellwald S, Panos L, Piechowiak E, et al. Predictors of unexpected early reocclusion after successful mechanical thrombectomy in acute ischemic stroke patients. Stroke. 2018;49:2643–51.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Unrelated: Dr Gralla reports acting as a global principal investigator of the STAR study; a clinical events committee member of the PROMISE study (Penumbra); and the principal investigator for the SWIFT DIRECT study (Medtronic). Dr Gralla also reports serving as a consultant for Medtronic and receiving Swiss National Science Foundation grants for magnetic resonance imaging in stroke. Professor Fischer is a global PI for the SWIFT DIRECT study (Medtronic) and receives research grants from SNSF. Professor Arnold has received speaker honoraria from Bayer, Boehringer Ingelheim, and Covidien, and scientific advisory board honoraria from Bayer, Boehringer Ingelheim, BMS, Pfizer, Covidien, Daichy Sankyo, and Nestlé Health Science. Dr Mosimann receives research grants from SNSF to study new therapeutic options for cerebral aneurysms. Mr Kaesmacher receives research grants from the Swiss Stroke Society and the Bangerter Foundation. All other authors have nothing to disclose.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed Consent
This study has obtained IRB approval from (indicate the relevant board), and the need for informed consent was waived. The registry was approved by the local ethics committee (Kantonale Ethikkommission für die Forschung Bern, Bern, Switzerland, Amendment Access Number: 231/2014).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dobrocky, T., Kaesmacher, J., Bellwald, S. et al. Stent-Retriever Thrombectomy and Rescue Treatment of M1 Occlusions Due to Underlying Intracranial Atherosclerotic Stenosis: Cohort Analysis and Review of the Literature. Cardiovasc Intervent Radiol 42, 863–872 (2019). https://doi.org/10.1007/s00270-019-02187-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-019-02187-9