Abstract
Purpose
To retrospectively compare early response to yttrium-90 radioembolization (Y90) according to volumetric iodine uptake (VIU) changes, Response Evaluation Criteria In Solid Tumor 1.1 (RECIST 1.1) and modified RECIST (mRECIST) in patients with intermediate-advanced hepatocellular carcinoma (HCC) and to explore their association with survival.
Materials and Methods
Twenty-four patients treated with Y90 and evaluated with dual-energy computed tomography before and 6 weeks after treatment were included. VIU was measured on late arterial phase spectral images; 6-week VIU response was defined as: complete response (CR, absence of enhancing tumor), partial response (PR, ≥ 15% VIU reduction), progressive disease (PD, ≥ 10% VIU increase) and stable disease (criteria of CR/PR/PD not met). RECIST 1.1 and mRECIST were evaluated at 6 weeks and 6 months. Responders included CR and PR. Overall survival (OS) was evaluated by Kaplan–Meier analysis and compared by Cox regression analysis.
Results
High intraobserver and interobserver agreements were observed in VIU measurements (k > 0.98). VIU identified a higher number of responders (18 patients, 75%), compared to RECIST 1.1 (12.5% at 6 weeks and 23.8% at 6 months) and mRECIST (29.2% at 6 weeks and 61.9% at 6 months). There was no significant correlation between OS and RECIST 1.1 (P = 0.45 at 6 weeks; P = 0.21 at 6 months) or mRECIST (P = 0.38 at 6 weeks; P = 0.79 at 6 months); median OS was significantly higher in VIU responders (17.2 months) compared to non-responders (7.4 months) (P = 0.0022; HR 8.85; 95% CI 1.29–88.1).
Conclusion
VIU is highly reproducible; as opposite to mRECIST and RECIST 1.1, early VIU response correlates with OS after Y90 in intermediate-advanced HCC patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the last decade, modified radiological criteria have been proposed for the assessment of tumor response to treatment in hepatocellular carcinoma (HCC), based on bidimensional or unidimensional measurement of persistent arterially enhancing tumor. Both European Association for the Study of the Liver (EASL) criteria [1] and modified Response Evaluation Criteria In Solid Tumors (mRECIST) [2] have been validated after percutaneous ablation and trans-arterial chemoembolization [3,4,5,6], and mRECIST are today recommended for HCC response assessment [7].
However, the usefulness of these criteria after yttrium-90 selective internal radiation therapy (Y90) is controversial [8,9,10,11]. Several studies have proposed alternative response criteria, based on volumetric measurements and/or functional parameters derived from perfusion and diffusion imaging [12,13,14,15,16,17]. Although much sophisticated software is being developed allowing volumetric analysis of perfusion and diffusion metrics [14, 15, 18,19,20,21,22], in routine clinical practice functional parameters are measured on single regions of interest (ROIs), thus being poorly reproducible [23] and potentially not representative of the entire tumor burden.
Over the last years, dual-energy computed tomography (DECT) has gained clinical acceptance. DECT can differentiate and quantify the iodine content that represents a surrogate biomarker of tumor vascularity. Gordic et al. [24] demonstrated a significant intra-individual correlation between CT arterial perfusion (AP) and iodine density obtained from DECT. Since early AP variations are correlated to survival in liver tumors [25], a similar relation could be postulated using the iodine density measured by DECT.
Compared to perfusion CT, DECT offers several advantages. It does not require additional contrast administration and radiation exposure, and, by using commercially available software, it enables iodine quantification in a segmented volume, obtaining the so-called volumetric iodine uptake (VIU), that could represent an appealing tool for quantitative response assessment [26].
Purpose of this retrospective preliminary single-center study was to compare early response to Y90 in terms of VIU changes, RECIST 1.1 and mRECIST in patients with intermediate-advanced HCC and to explore the association of these criteria with survival.
Materials and Methods
Institutional review board approved the study, and written informed consent was waived. All patients provided written informed consent for CT examinations and Y90.
We retrospectively analyzed the data of HCC patients treated with Y90 from February 2013 (date of installation of DECT equipment) to December 2015, evaluated with DECT and spectral imaging less than 1 month before the procedure and 6 ± 2 weeks (mean 6.2 ± 1.7) after treatment.
The inclusion criteria were as follows:
-
naïve HCC diagnosed according to international guidelines [27] and excluded from curative treatments and trans-arterial chemoembolization (TACE; main exclusion criteria for TACE represented by macrovascular invasion and/or tumors larger than 10 cm) after multidisciplinary tumor board discussion;
-
preserved liver function (Child–Pugh A or B7);
-
no contraindications to Y90 (e.g., severe allergy to contrast agents, hepato-pulmonary shunt > 20%, any other contraindication to angiography and selective catheterization).
Among 65 naïve HCC patients treated with Y90 during the study period, 41 patients did not undergo DECT with spectral imaging before and/or after treatment and were excluded from the study. The final population consisted of 24 patients (20 males; mean age 63.4 years), predominantly with liver cirrhosis (87.5%) and advanced-stage HCC (Barcelona Clinic of Liver Cancer, BCLC stage C, 87.5%) due to portal vein tumor thrombus (PVTT). Clinical and demographic data are summarized in Table 1.
Y90 Procedure
Y90 was preceded by a simulation procedure consisting of an angiographic study and a SPECT-CT examination after intraarterial injection of 99Tc-labeled macroaggregated albumin.
Dosimetry was obtained according to the manufacturers’ recommendation. Treatment was performed using resin (n = 22; Sir-Spheres®; Sirtex Medical Europe GmbH, Bonn, Germany) or glass particles (n = 2; TheraSphere®; Biocompatibles UK Ltd, Surrey, UK) labeled with Y90. Treatment was lobar in 19 (79.2%) cases, bi-segmental in two (8.3%) cases and segmental in three (12.5%) patients. The median treatment activity delivered was 1.25 GBq (range 0.35–4 GBq). The median dose delivered was 100 Gy (range 90–120 Gy). All the procedures were completed successfully, delivering the entire planned dose.
CT Protocol
CT protocol (Discovery CT750 HD system; GE Healthcare, Wisconsin, USA) included an unenhanced scan followed by tri-phasic acquisition, after injection of 110 mL non-ionic contrast medium (Iomeron 400; Bracco, Milan, Italy) through antecubital venous access at a flow rate of 4 mL/s, followed by 40 mL saline flush at the same flow rate, using an automatic injector (MEDRAD Stellant; MEDRAD, INC., Warrendale, PA, USA). Post-contrast imaging scan delay was determined by automated bolus triggering (SmartPrep; GE Healthcare). Late arterial phase was acquired in spectral imaging mode (Supplementary Table 1).
Image Analysis
On an independent workstation (Advantage Windows 4.6, GE Healthcare), two independent radiologists (IB and FT, 13 and 10 years of experience) calculated VIU in treated liver lobe before and after treatments. Readers were allowed to compare the late arterial phase with the portal venous and delayed phases to recognize “viable” tumor, defined as the hyperattenuating areas in the arterial phase with washout in the subsequent phases. VIU measurement was taken by semi-automatic segmentation of the entire tumor load on the late arterial phase, including macrovascular tumor thrombosis, through manual definition of the margins of the enhancing tumor on the axial slices. Subtle and patchy hypoattenuating areas detected in the context of enhancing tumor were included in the segmented volume. Once the segmentation was completed, the software automatically calculated the overall tumor volume (cm3) and its mean iodine content (μg/cm3). The total VIU (mg) was obtained with the following formula: [(lesion volume × mean iodine concentration)/1000]. Segmentation was repeated three times, at an interval of at least 1 month, and the mean values were used for further analysis.
In the lack of published data, VIU response criteria were defined according to Dai et al. [26]: complete response (CR, complete disappearance of arterially enhancing tumor), partial response (PR, reduction of VIU of at least 15%), progressive disease (PD, increase in VIU of at least 10%) and stable disease (SD, criteria of CR/PR/PD not met).
Another radiologist (LC, 13 years of experience), blinded to VIU findings and original reports, retrospectively evaluated target tumor response within the treated liver lobe, at 6-week and 6-month follow-up, according to RECIST 1.1 [29] and mRECIST [2]. Response assessment was compared to the original report; in case of discrepancy, consensus was reached between three radiologists (LC, EB, RC).
For all criteria, responders included CR and PR.
Statistical Analysis
Data were analyzed using descriptive statistics (mean and standard deviation, SD) and compared with Chi square or Fisher exact test for categorical data and Student’s t test for paired data. Intra- and interobserver agreements in VIU measurement were tested by Bland and Altman plot.
Survival curves were calculated as the time between first treatment and death or end of follow-up (December 15th, 2017) using Kaplan–Meier analysis, and compared with Wilcoxon signed rank test. Cox proportional hazards were used to test the association between overall survival (OS) and baseline factors and response criteria.
Statistical analysis was performed with dedicated software (SAS, Cary, NC USA) considering a P value < 0.05 as statistically significant.
Results
Radiological Tumor Response
The mean VIU values were 10.8 ± 12 mg before treatment and 8.1 ± 13.4 mg after treatment, with a mean VIU reduction of 15.7% (median 55.4%; 95% CI − 33 to 64.4). Almost perfect intraobserver and interobserver agreements were observed in VIU measurements (Table 2).
By VIU criteria, PR was observed in 18 patients (75%) and PD in six cases (25%). Association between VIU response and baseline parameters is presented in Supplementary Table 2.
RECIST 1.1 and mRECIST responses are reported in Table 3. Three patients died within 6 months after treatment; thus, the 6-month CT control was available in 21 patients. Out of 90 retrospective assessments, consensus reading was required in five cases (5.6%).
At 6 weeks, by RECIST 1.1 three cases (12.5%) were considered PR, while 75% of cases were SD. According to mRECIST, seven (29.2%) patients were defined as responders with four cases (16.7%) of CR. Six-month CT controls demonstrated an increased number of responders, with 7 (33.3%) CR and six (28.6%) PR according to mRECIST, and 5 (23.8%) PR according to RECIST 1.1. Using two-tailed Fisher’s exact test, no statistically significant correlation was found between early VIU response and RECIST 1.1 (P = 0.13 at 6 weeks; P = 0.19 at 6 months) or mRECIST (P = 0.55 at 6 weeks; P = 0.64 at 6 months). Of notice, 11 cases of SD according to mRECIST and 15 cases according to RECIST 1.1 were defined as responders by VIU at early follow-up (Figs. 1, 2).
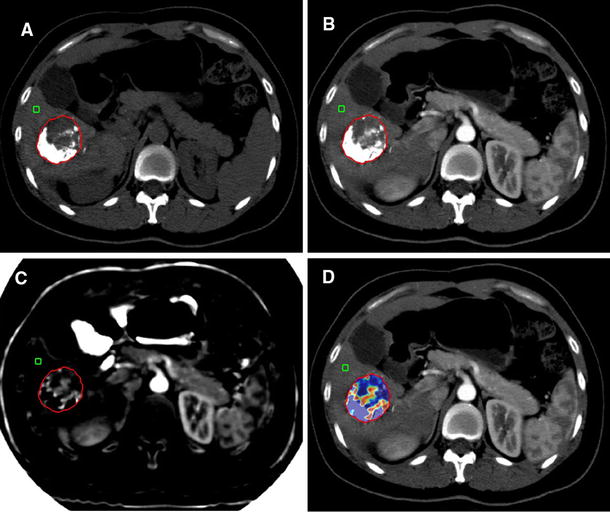
74-Year-old female patient with large, single HCC nodule in segment 1 evaluated before (axial scan arterial phase, A; VIU measurement, B) and 6 weeks after (axial scan arterial phase, C; VIU measurement, D) Y90 radioembolization. Early after treatment, the lesion does not show well-defined areas of necrosis; the maximum axial diameter is reduced by 11.5% (A, C); thus, the case is defined as SD according to RECIST and mRECIST. VIU (B, D; ROI1: volume; Av: average iodine content) is reduced by 43.7% (from 8.07 to 4.54 mg); thus, the patient is considered as VIU responder. The patient died 17 months after Y90
75-Year-old male patient with bioptically proven HCC invading the right portal vein and extending into the left portal vein; CT images performed before (late arterial phase, A; portal venous phase, B; VIU measurement C) and after (late arterial phase, D; portal venous phase, E; VIU measurement, F) Y90. After treatment, at visual assessment, the extension of portal vein infiltration is reduced with concomitant reduction in arterial enhancement. However, as portal vein thrombosis is a non-target lesion, the patient was considered stable both by RECIST 1.1 and mRECIST. VIU is reduced from 3.33 to 0.52 mg (reduction of 84%), defining the patient as responder. The patient died 17.5 months after Y90
Overall Survival
One patient was lost to follow-up 9 months after Y90. The remaining patients were followed until death or end of follow-up (December 15th, 2017). At latest follow-up, 20 patients (83.3%) had died, with a median survival of 11 months (95% CI 7.9–29.4).
Among all baseline factors, OS was significantly associated with Child–Pugh score and AFP values (Table 4).
There was no significant correlation between OS and RECIST 1.1 (P = 0.45 at 6 weeks and P = 0.21 at 6 months; Fig. 3A, B) or mRECIST (P = 0.38 at 6 weeks and P = 0.79 at 6 months; Fig. 3C, D). Conversely, VIU response was significantly associated with survival (P = 0.0022), with a median survival of 7.4 months (95% CI 3.1–10.7) in non-responders and 17.2 months in responders (95% CI 10.8–41) (Fig. 3E).
Kaplan–Meier curves comparing overall survival between responders and non-responders according to RECIST 1.1 at 6 weeks (A) and at 6 months (B), mRECIST at 6 weeks (C) and at 6 months (D) and VIU (E). Only VIU response showed a statistically significant association with overall survival (P = 0.0026)
Factors identified at univariate analysis (Child–Pugh score, AFP and VIU response) were confirmed to be independently associated with survival in the multivariate model (Table 4).
Discussion
In the present study, a new criterion was investigated that combines volumetry and iodine content measurement as the expression of tumor vascularity. Our preliminary results demonstrate that VIU can represent a reliable tool for the early assessment of tumor response after Y90.
RECIST 1.1 have a limited role in HCC response assessment [30], and mRECIST are currently recommended by European guidelines for HCC follow-up [7].
In a radiological–pathological analysis of 15 patients transplanted after Y90 with or without, Vouche et al. [10] demonstrated that, although EASL and mRECIST detected a higher number of responders compared to WHO and RECIST, none of these criteria could reliably predict complete pathological necrosis.
The limitations of RECIST and mRECIST are partially related to the treatment’s nature. The main effect of Y90 is the exposure to very high radiation doses, while the embolic effect is minimal, particularly when using glass spheres compared to resin spheres. Hence, at early follow-up tumor necrosis may not be macroscopically evident or may be inhomogenously distributed, and, therefore, difficult to quantify, despite effective therapy. Moreover, radiation-induced inflammation is often appreciated in the treated area as arterially enhancing tissue that may be difficult to differentiate from “viable” tumor [8,9,10, 31].
HCC stage may also play a role in defining usefulness of size criteria. A recent study on 134 BCLC A patients with single HCC demonstrated that EASL criteria outperform RECIST 1.1 in predicting survival [32]. However, Y90 is frequently indicated in patients with PVTT and infiltrative HCC [33,34,35,36], which are defined non-measurable and non-target lesions (Fig. 2) [2, 29]. Thus, in more advanced stages, a relatively high number of patients will be classified as having SD at early follow-up using conventional criteria. In our series, 54.2 and 70.8% of patients were defined as SD with mRECIST and RECIST 1.1, respectively, while the majority of these SD cases were VIU responders.
Functional biomarkers derived from perfusion and diffusion imaging are under investigation for tumor response assessment [15, 16, 25, 37]. However, they are usually calculated on single axial planes and may not be representative of the entire tumor volume. In the future, volumetric measurement of diffusion and/or perfusion parameters could become the reference for tumor response evaluation [20,21,22,23]. However, these measurements require complex algorithms and dedicated software that are not yet widely available.
DECT is gaining acceptance as a valid technology providing high-quality images and allowing identification of different materials [38], with promising clinical applications. By dedicated software provided by the manufacturer, iodine content can be measured within a segmented volume, representing an appealing biomarker of tumor vascularity. Iodine uptake has been investigated in patients under sorafenib [26], and it has been demonstrated to be significantly associated with arterial perfusion [24]. Compared to perfusion CT, it does not require additional acquisitions and contrast administrations, representing an interesting tool in daily clinical practice. Moreover, VIU is a reproducible quantitative parameter, as demonstrated by the excellent intra- and interobserver agreement in our study.
Our results show that, using the VIU cutoff value proposed by Dai et al. [26], it is possible to identify a higher number of responders compared to RECIST 1.1 and mRECIST at early follow-up after Y90 and that VIU response has a significant correlation with OS, as opposite to mRECIST and RECIST 1.1.
In view of the limitations of conventional size criteria in the early follow-up after Y90, most experts suggest that response should be assessed 3–6 months after treatment [32]. In the present study, 6-month RECIST 1.1 and mRECIST responses were not significantly associated with OS, despite the higher number of responders. Besides, evaluating tumor response later on follow-up implies a minimum time of 6–9 months before considering any other therapeutic approach in non-responders, with a dropout risk due to clinical deterioration and tumor progression. A much sensitive response parameter (such as VIU) used early after Y90 might allow prompt recognition of non-responders, who may be considered for alternative treatments.
Our study is limited by the retrospective design and the low number of patients. Population was heterogeneous in terms of clinical and tumor characteristics, influencing our results. Although well-known prognostic factors such as Child–Pugh score and AFP values were confirmed in our study, other parameters, such as BCLC stage and PVTT extent, were not identified as significantly associated with OS, because of the small sample size. Due to the limited sample size, VIU cutoff values were not calculated by statistical means and were derived from the only publication proposing VIU response criteria in HCC patients [26]. Although these criteria seem to be reliable, given the significant association with OS, prospective larger studies are needed to calculate VIU cutoff values. Finally, mRECIST and RECIST were evaluated by a single radiologist; however, results were compared to the original reports and, in case of disagreement, consensus was reached.
Despite these limitations, our preliminary study demonstrates that, while mRECIST and RECIST 1.1 fail to demonstrate a correlation with post-treatment OS, VIU may represent a highly reproducible and useful tool for the early assessment of tumor response in intermediate-advanced HCC patients treated with Y90. Prospective studies are needed to confirm our preliminary findings.
References
Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–30.
Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60.
Gillmore R, Stuart S, Kirkwood A, et al. EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization. J Hepatol. 2011;55:1309–16.
Shim JH, Lee HC, Kim SO, et al. Which response criteria best help predict survival of patients with hepatocellular carcinoma following chemoembolization? A validation study of old and new models. Radiology. 2012;262:708–18.
Jung ES, Kim JH, Yoon EL, et al. Comparison of the methods for tumor response assessment in patients with hepatocellular carcinoma undergoing transarterial chemoembolization. J Hepatol. 2013;58(6):1181–7.
Vincenzi B, Di Maio M, Silletta M, et al. Prognostic relevance of objective response according to EASL criteria and mRECIST criteria in hepatocellular carcinoma patients treated with loco-regional therapies: a literature-based meta-analysis. PLoS ONE. 2015;10(7):0133488.
European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer. EASL–EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–43.
Atassi B, Bangash AK, Bahrani A, et al. Multimodality imaging following 90Y radioembolization: a comprehensive review and pictorial essay. Radiographics. 2008;28:81–99.
Ibrahim SM, Nikolaidis P, Miller FH, et al. Radiologic findings following Y90 radioembolization for primary liver malignancies. Abdom Imaging. 2009;34:566–81.
Vouche M, Kulik L, Atassi R, et al. Radiological-pathological analysis of WHO, RECIST, EASL, mRECIST and DWI: imaging analysis from a prospective randomized trial of Y90 ± sorafenib. Hepatology. 2013;58:1655–66.
Seyal AR, Gonzalez-Guindalini FD, Arslanoglu A, et al. Reproducibility of mRECIST in assessing response to transarterial radioembolization therapy in hepatocellular carcinoma. Hepatology. 2015;62:1111–21.
Galizia MS, Töre HG, Chalian H, et al. MDCT necrosis quantification in the assessment of hepatocellular carcinoma response to yttrium 90 radioembolization therapy: comparison of two-dimensional and volumetric techniques. Acad Radiol. 2012;19:48–54.
Duke E, Deng J, Ibrahim SM, et al. Agreement between competing imaging measures of response of hepatocellular carcinoma to yttrium-90 radioembolization. J Vasc Interv Radiol. 2010;21:515–21.
Chapiro J, Lin M, Duran R, et al. Assessing tumor response after loco-regional liver cancer therapies: the role of 3D MRI. Expert Rev Anticancer Ther. 2015;15:199–205.
Reiner CS, Gordic S, Puippe G, et al. Histogram analysis of CT perfusion of hepatocellular carcinoma for predicting response to transarterial radioembolization: value of tumor heterogeneity assessment. Cardiovasc Interv Radiol. 2016;39:400–8.
Kokabi N, Camacho JC, Xing M, et al. Apparent diffusion coefficient quantification as an early imaging biomarker of response and predictor of survival following yttrium-90 radioembolization for unresectable infiltrative hepatocellular carcinoma with portal vein thrombosis. Abdom Imaging. 2014;39:969–78.
Weng Z, Ertle J, Zheng S, et al. Choi criteria are superior in evaluating tumor response in patients treated with transarterial radioembolization for hepatocellular carcinoma. Oncol Lett. 2013;6:1707–12.
Vouche M, Salem R, Lewandowski RJ, et al. Can volumetric ADC measurement help predict response to Y90 radioembolization in HCC? Abdom Imaging. 2015;40:1471–80.
Tacher V, Lin M, Duran R, et al. Comparison of existing response criteria in patients with hepatocellular carcinoma treated with transarterial chemoembolization using a 3D quantitative approach. Radiology. 2016;278:275–84.
Bonekamp S, Halappa VG, Geschwind JF, et al. Unresectable hepatocellular carcinoma: MR imaging after intraarterial therapy. Part II. Response stratification using volumetric functional criteria after intraarterial therapy. Radiology. 2013;268:431–9.
Rathmann N, Budjan J, Mari F, et al. Semiautomatic whole-lesion apparent diffusion coefficient assessment for early prediction of liver tumor response to radioembolization. Anticancer Res. 2016;36:2961–6.
Zhu X, Sobhani F, Xu C, et al. Quantitative volumetric functional MR imaging: an imaging biomarker of early treatment response in hypo-vascular liver metastasis patients after yttrium-90 transarterial radioembolization. Abdom Radiol (NY). 2016;41:1495–504.
Bonekamp D, Bonekamp S, Halappa VG, et al. Interobserver agreement of semi-automated and manual measurements of functional MRI metrics of treatment response in hepatocellular carcinoma. Eur J Radiol. 2014;83:487–96.
Gordic S, Puippe GD, Krauss B, et al. Correlation between dual-energy and perfusion CT in patients with hepatocellular carcinoma. Radiology. 2016;280:78–87.
Reiner CS, Morsbach F, Sah BR, et al. Early treatment response evaluation after yttrium-90 radioembolization of liver malignancy with CT perfusion. J Vasc Interv Radiol. 2014;25:747–59.
Dai X, Schlemmer HP, Schmidt B, et al. Quantitative therapy response assessment by volumetric iodine-uptake measurement: initial experience in patients with advanced hepatocellular carcinoma treated with sorafenib. Eur J Radiol. 2013;82:327–34.
Bruix J, Sherman M, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2.
Shi J, Lai EC, Li N, et al. Surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol. 2010;17:2073–80.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Forner A, Ayuso C, Varela M, et al. Locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable? Cancer. 2009;115:616–23.
Keppke AL, Salem R, Reddy D, et al. Imaging of hepatocellular carcinoma after treatment with yttrium-90 microspheres. AJR Am J Roentgenol. 2007;188:768–75.
Riaz A, Gabr A, Abouchaleh N, et al. Radioembolization for hepatocellular carcinoma: statistical confirmation of improved survival in responders by landmark analyses. Hepatology. 2018;67:873–83.
Mazzaferro V, Sposito C, Bhoori S, et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology. 2013;57:1826–37.
Sangro B, Carpanese L, Carpanese L, Cianni R, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011;54:868–78.
De la Torre M, Buades-Mateu J, de la Rosa PA, et al. A comparison of survival in patients with hepatocellular carcinoma and portal vein invasion treated by radioembolization or Sorafenib. Liver Int. 2016;36:1206–12.
Salem R, Lewandowski RJ, Kulik L, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140:497–507.
Rhee TK, Naik NK, Deng J, et al. Tumor response after yttrium-90 radioembolization for hepatocellular carcinoma: comparison of diffusion-weighted functional MR imaging with anatomic MR imaging. J Vasc Interv Radiol. 2008;19:1180–6.
Agrawal MD, Pinho DF, Kulkarni NM, et al. Oncologic applications of dual-energy CT in the abdomen. Radiographics. 2014;34:589–612.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Irene Bargellini received honoraria from GE Healthcare, Biocompatibles UK LTD, Sirtex, Bayer Spa; Laura Crocetti received honoraria from GE Healthcare; the remaining authors have no conflict of interests to declare.
Ethical Approval
All procedures performed in the study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments.
Informed Consent
For this type of study, informed consent was waived by the Local Ethical Committee.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bargellini, I., Crocetti, L., Turini, F.M. et al. Response Assessment by Volumetric Iodine Uptake Measurement: Preliminary Experience in Patients with Intermediate-Advanced Hepatocellular Carcinoma Treated with Yttrium-90 Radioembolization. Cardiovasc Intervent Radiol 41, 1373–1383 (2018). https://doi.org/10.1007/s00270-018-1962-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-018-1962-8