Abstract
Purpose
To evaluate the value of quantitative volumetric functional MR imaging in early assessment of response to yttrium-90-labeled (90Y) transarterial radioembolization (TARE) in patients with hypo-vascular liver metastases.
Materials and methods
Seventy four metastatic lesions in 14 patients with hypo-vascular liver metastases after TARE were included in this retrospective study. Diffusion and contrast-enhanced MR imaging was performed before and early after treatment. All MR images were analyzed by two experienced radiologists. Response by anatomic metrics (RECIST, mRECIST, EASL) and functional metrics (ADC and arterial and venous enhancement) were reported in targeted and non-targeted lesions. A two-sample paired t test was used to compare the changes after TARE. A p value of <0.05 was considered statistically significant.
Results
The anatomic metrics did not show any significant changes in both targeted and non-targeted groups. Targeted lesions demonstrated an increase in mean volumetric ADC (23.4%; p = 0.01), a decrease in arterial and venous enhancement (−22.9% and −6.7%, respectively; p < 0.001 and p = 0.002, respectively) 1 month after treatment. Twenty one responding lesions (42%) by RECIST at 6 months demonstrated a significant increase in volumetric ADC (37.2%; p = 0.01), decrease in arterial and venous enhancement (−58.5% and −23.9%, respectively; p < 0.001) at 1 month post-treatment. Responding lesions did not change significantly by anatomic metrics.
Conclusions
RECIST, mRECIST, and EASL criteria failed to stratify lesions into responders and non-responders early after TARE in hypo-vascular liver metastasis. Quantitative volumetric functional MR imaging could be a promising tool as a biomarker for predicting early response and can potentially be utilized in clinical trials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The liver is a common site for primary and secondary malignancies. Hepatic metastases are found in 40% of all patients diagnosed with cancer [1]. Autopsy studies have demonstrated that the liver is involved in 50% to 70% of metastases from melanoma, lymphoma, and common malignancies that originate in the breast, lung, and gastrointestinal tract. Based on their blood supply, metastases may be categorized as hyper-vascular and hypo-vascular. Colon, breast, lung, and gastric carcinomas are the most common tumors causing hypo-vascular liver metastases, which typically show minimal rim or perilesional enhancement [2]. Although surgical resection is the only curative therapeutic option for secondary hepatic malignancies, most patients are often poor candidates for this treatment because of several factors including poor hepatic reserve, the presence of numerous bilobar tumors or extrahepatic disease. For those patients, intraarterial therapy is becoming an essential therapeutic option [3].
Offering a minimally invasive treatment pathway with promising clinical results, transarterial radioembolization (TARE) with Yttrium-90 (90Y) microspheres is a catheter-based, liver-directed therapy that is now available for the treatment of primary and metastatic liver malignancy ineligible to undergo surgery [4–8]. Delivered intraarterially through a catheter placed in the hepatic artery feeding the tumors, the microspheres provide a lethal radiation dose to tumor cells, destroying vascular flow and resulting in cellular death and tumor necrosis [9], with low toxicity to normal liver and minimal side effects.
Precise and early assessments of treatment response after 90Y microsphere is critical not only to evaluate the therapeutic effect, but also to determine whether repeated treatment is needed [10]. Current traditional and accepted anatomic imaging criteria for evaluating tumor response include response evaluation criteria in solid tumors (RECIST), which requires uni-dimensional measurement of the entire lesion in an axial plane. The European association for the study of the liver (EASL) proposed the estimation of reduction in viable tumor area bi-dimensionally [4]. More recently, modified RECIST (mRECIST) was proposed, and is based on reduction of the longest diameter of enhancing tumor in the axial plane. Measuring the residual enhancing tissue by EASL and mRECIST criteria was intended to predict treatment response of hyper-vascular tumors, particularly hepatocellular carcinoma (HCC). The utility of these anatomic metrics in assessing response of hypo-vascular metastases to TARE is not well evaluated.
The recent development of novel functional magnetic resonance (MR) imaging techniques provided objective, quantitative evaluation of tissue perfusion and cellular integrity. Diffusion-weighted imaging (DWI) and the quantifiable apparent diffusion coefficient (ADC) detect motion of water within tumor tissues, which has been useful in tumor detection, characterization, and in monitoring response to treatment [11–13]. Previous studies have demonstrated that volumetric functional MR imaging can help in accurately assessing tumor response after loco-regional therapy (LRT) in patients with HCC, neuroendocrine liver metastases (NELM), and islet cell liver metastases (ICLM) [14–16]. The objective of this study was to assess the value of quantitative volumetric biomarkers to evaluate early (1 month) response after 90Y-radioembolization in hypo-vascular liver metastasis, and compare those metrics to current metrics including RECIST, mRECIST, and EASL.
Materials and methods
This single-institute retrospective study was performed in compliance with the Health Insurance Portability and Accountability Act (HIPAA) after obtaining a waiver for informed consent approved by our institutional review board (IRB).
Study population
We reviewed a database of patients with hypo-vascular metastatic liver tumors with absent or limited extrahepatic disease who underwent first line LRT from January 2008 to March 2015 at our Institution. All patients enrolled had hypo-vascular liver metastases by MRI described as hypo-intense appearance compared to the enhancing normal liver parenchyma with perilesional enhancement. Inclusion criteria for enrollment were (a) patients who received a clinical confirmed diagnosis of liver metastases from a primary malignancy; (b) patients who have undergone 90Y microsphere treatment at our institution; and (c) patients who underwent MR imaging with DWI and contrast-enhanced MR imaging at baseline, 1 and 6 months after treatment. Of 44 patients who had hypo-vascular liver metastases, we excluded 27 patients who undergone TACE treatment, and 3 patients for the absence of MR images before or after treatment. The remaining 14 patients were included in this retrospective study (Fig. 1).
90Y microsphere treatment
90Y is a beta radiation emitter with half-life of 64.2 h and an average energy of 0.94 MeV. The radiation had a mean tissue penetration of 2.5 mm, and 94% of the dose is delivered within the first 11 days following administration of TheraSpheres (BTG International Canada Inc, Ottawa, ON, Canada). TheraSpheres are made of glass and 90Y is embedded within the glass matrix leading to higher density and specific activity (2500 Bq) of the preparation [9].
All procedures were performed by an experienced multidisciplinary team including board-certified interventional radiologists. A week before treatment, each patient underwent an angiogram to map the anatomy of the hepatic arterial system and protectively embolized vessels permitting microspheres to enter the GI tract. The liver volume treated was determined by MRI with a conversion factor of 1.03 g/cm3, the corresponding liver mass was determined. A technetium-99 (99Tc) macroaggregated albumin scan was performed to observe if there was gastrointestinal flow and to determine the percent of injected activity shunted to the lungs. According to individual patient clinical demand, the elective segmental or lobar TARE was injected into the hepatic artery feeding the tumor. Patients were monitored for 2 h after the procedure and were subsequently discharged [17, 18].
MR imaging technique and parameters
All 14 patients underwent MR scan at baseline and follow-up at 1 and 6 months after TARE. MRI was performed with a 1.5 T whole-body MR (Magnetom Avanto; Siemens Healthcare, Erlangen, Germany) unit using a phased array torso coil. The imaging protocol included axial T2-weighted fast spin-echo (FSE) navigation MR imaging (TR/TE 4500/92 ms; 256 × 256 matrix; 8-mm-thick slice; 2 mm gap; 32 Hz/pixel bandwidth); axial breath-hold single-shot echo-planar (SE-EPI) DWI (TR/TE 3000/69 ms, 128 × 128 matrix, 380 mm field of view, 8-mm-thick slice, 2 mm gap, b values of 0 and 750 mm2/s, 64 Hz/pixel bandwidth) with fat suppression; and axial breath-hold unenhanced and contrast-enhanced (0.1 mmol/kg intravenous gadopentetate dimeglumine, Magnevist; Bayer, NJ) T1-weighted three-dimensional fat-suppressed spoiled gradient-echo (GRE) imaging (TR/TE 5.77/2.77 ms, 400 mm field of view, 192 × 160 matrix, 2.5 mm slice thickness, 64 Hz/pixel bandwidth, 10° flip angle) in hepatic arterial phase (20 s) and portal venous phases (70 s) (Table 1).
MR image analysis
All MR images were evaluated by consensus of two experienced radiologists. Up to 5 hypo-vascular metastatic lesions were selected with a diameter ≥10 mm per patient in both targeted and non-targeted lobes to ensure independent sampling. Smaller (<10 mm) lesions were not included due to difficulty in measuring ADC values of these lesions. Also, lesions close to the diaphragm were excluded to avoid breathing artifacts (Fig. 1).
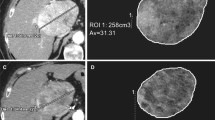
A total of 50 targeted and 24 non-targeted lesions were analyzed at baseline and 1 month post-treatment according to (a) RECIST, (b) mRECIST, (c) EASL criteria, (d) volumetric ADC, and (e) volumetric HAE and PVE. Conventional anatomical metrics, including RECIST, mRECIST, and EASL were measured in portal venous phase using Emageon Ultravisual (Medical Systems Corporation, Birmingham, AL) software. Volumetric ADC and enhancement analysis was performed using proprietary software (MR Oncotreat, Siemens Healthcare, Princeton, USA). In the first step, patient DICOM data were imported into the software and registered for calculation; secondly, tumor borders were defined with an interactive segmentation technique that was previously described [19]. Percent change in volumetric tumor ADC and enhancement at 1 month follow-up compared with baseline values was calculated using the following formula:\( \frac{{T_{\text{post}} - T_{\text{pre}} }}{{T_{\text{pre}} }} \times 100 \), where T pre is volumetric ADC or enhancement before treatment and T post is volumetric ADC or enhancement 1 month after TARE.
Study endpoint
RECIST at 6 months after initial TARE was our endpoint for each lesion. Fifty targeted lesions were stratified into responders [including complete response (CR) and partial response (PR)] and non-responders [including stable disease (SD) or progressive disease (PD)], as defined in Table 2.
Statistical analysis
A two-sample paired t test was used to determine if comparison between anatomic and volumetric functional MR metrics at baseline and 1 month after TARE were significantly different for all targeted and non-targeted lesions, including tumor size by RECIST, tumor enhancement by mRECIST, tumor enhancement by EASL, volumetric HAE, volumetric PVE, and volumetric ADC. Similar comparisons were also made for responders and non-responders. Statistical analysis was performed with a software package (SPSS, version 16.0; SPSS, Chicago, IL). A p value of <0.05 was considered significant.
Results
Demographic information
Demographic information of the 14 patients (mean age 60 ± 13 years; range between 33 and 86 years) in our study is summarized in Table 3. A total of 50 targeted and 24 non-targeted lesions were analyzed. The majority of patients included were Caucasian (43%) and females (86%).
90Y microsphere treatment
All 14 patients successfully completed 90Y microsphere radioembolization treatment without immediate (within 24 h) complications. Five patients underwent TARE once, 8 patients underwent TARE twice, and 1 patient underwent TARE three times (mean 1.7 ± 0.6, range 1–3). Nine patients received TARE in both lobes, 4 patients to the right lobe, and 1 patient to the left lobe.
MR imaging findings
The mean number of lesions for the 14 patients was 3.5. The mean pretreatment diameter was 2.9 ± 2.4 and 2.6 ± 1.3 cm for the targeted and non-targeted lesions, respectively.
Anatomic and volumetric functional metrics at baseline and 1 month post-treatment in targeted lesions
Table 4 shows anatomic and volumetric measurements for the targeted lesions at baseline and 1 month after treatment. Targeted lesions did not change significantly in size by RECIST (p = 0.74), mRECIST (p = 0.31), nor EASL (p = 0.23). Targeted tumors demonstrated a significant decrease in mean HAE of 22.9% (p < 0.001), a decrease in mean PVE of 6.7% (p = 0.002), and a significant increase in mean ADC value of 23.4% (p = 0.01) (Fig. 2).
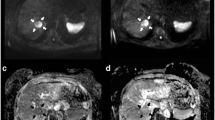
Anatomic metrics in a 60-year-old female diagnosed with breast cancer liver metastases. RECIST was measured in an axial plane in portal venous phase images at baseline (A), 1 month (B), and 6 months (C) post-treatment (arrows). The maximum diameter of the lesion did not show any changes in size, the lesion measured 1.52 cm at baseline and at 6 month follow-up. The lesions were categorized as SD by RECIST. Volumetric metrics of the tumor at baseline (D) and 1 month after treatment (E) in the same lesion. Enhancement in the hepatic arterial phase decreased from 60.9% (F) to 6.1% (G), showing a 90% decreased enhancement. Portal venous phase enhancement decreased from 100% (H) to 57.6% (I), demonstrating a 46.6% decreased enhancement. ADC changed from 0.75 × 10−3 mm2/s (J) to 2.64 × 10−3 mm2/s (K), increasing by 254%. The histogram (blue = pretreatment data, orange = post-treatment data) showed favorable response with leftward shift in the hepatic arterial (L) and portal venous (M) phases, and rightward shift in ADC (N). ADC images before (O) and after (P) therapy are also shown
Anatomic and volumetric functional metrics at baseline and 1 month post-treatment in non-targeted lesions
Table 5 shows anatomic and volumetric measurements for the non-targeted lesions at baseline and 1 month after treatment. Similar to targeted metastasis, non-targeted lesions did not change significantly in size by RECIST (p = 0.17), mRECIST (p = 0.30), nor EASL (p = 0.47). Also, functional volumetric measures of non-targeted lesions did not show significant changes by mean HAE (p = 0.30), mean PVE (p = 0.88), or mean ADC value (p = 0.72) after treatment.
Stratification of targeted lesions by RECIST response at 6-month follow-up
In accordance to RECIST guidelines at 6-month follow-up, the targeted lesions were stratified into 21 (42%) responders [CR 10% (n = 5); PR 32% (n = 16)] and 29 (58%) non-responders [SD 44% (n = 22); PD 14% (n = 7)].
Anatomic and volumetric functional metrics at 1 month post-treatment in targeted lesions
Table 6 shows anatomic and volumetric measurements for the 21 responders and 29 non-responders at baseline and 1 month after treatment. Responders did not change significantly in size by RECIST (p = 0.08), mRECIST (p = 0.08), nor EASL (p = 0.06). Responders demonstrated a significant decrease in mean HAE of 58.5% (p < 0.001), a significant decrease in mean PVE of 23.9% (p < 0.001), and a significant increase in mean ADC value of 37.2% (p = 0.01). Moreover, non-responders did not change significantly in size by RECIST (p = 0.08), mRECIST (p = 0.72), nor EASL (p = 0.90). Non-responders also did not show significant changes in mean HAE (increased by 2.9%, p = 0.10), mean PVE of (increased by 5.7%, p = 0.37), and ADC value of (increased by 7.4%, p = 0.31).
Discussion
To our knowledge, the value of quantitative volumetric functional MRI in predicting early treatment response of hypo-vascular hepatic metastasis has not been established to date. This retrospective study identified the potential value of quantitative volumetric functional MRI by ADC, HAE, and PVE to predict early (1 month) response to 90Y-radioembolization in hypo-vascular liver metastasis. Furthermore, anatomic methods (RECIST, m RECIST, EASL) failed to stratify lesions according to tumor response early after therapy.
90Y microspheres obliterate tumor vascular flow and result in cellular death and tumor necrosis, affecting the functional and physiologic behavior of the lesion more than the tumor size. Traditional anatomic criteria based on decrease in tumor size in the axial plane show significant limitation in detecting early tumor response to TARE therapy [18, 20]. While tumor shrinkage may not be initially apparent, there were changes in tumor ADC and enhancement that could be valuable in assessing early therapeutic efficacy [21–23]. Previous studies [4, 15, 24] demonstrated the promising role of quantitative volumetric functional MRI to detect early tumor response to LRT of liver malignancies. Changes in volumetric functional MR metrics may precede conventional tumor size changes, potentially overcoming the limitations of mRECIST and EASL.
Our study demonstrated statistically significant changes in functional MRI metrics (ADC, HAE, and PVE) early (1 month) after treatment in all 50 targeted lesions (Table 4). The anatomic metrics did not show any significant changes in both, the targeted and the non-targeted groups (Table 5). This finding is particularly important not only to evaluate the therapy effect, but also to determine whether repeated treatment is needed before disease progression is seen by anatomical metrics. Volumetric functional MR metrics provide accurate and objective assessment of early treatment response after 90Y microsphere, and could potentially be valuable in the clinical management of patients for prediction of therapeutic effect and in guiding future therapy [20]. Early responders by functional MR metrics may not require additional therapy unless disease progression is confirmed at follow-up imaging.
Our study also showed statistically significant changes in volumetric functional MRI metrics early (1 month) after treatment in 21 (42%) responders. For all responders, volumetric HAE and PVE decreased significantly (58.5% and 23.9% respectively), and volumetric ADC increased by 37.2% 1 month after TARE. On the contrary, anatomic metrics did not show any significant change in the responders or non-responders after therapy. Our results suggest that these potential novel functional MRI techniques can stratify patients as responders and non-responders early (1 month) after TARE, even before changes in tumor size and/or morphologic characteristics occur [16, 25].
Response by conventional size measurements may take several months to document after TARE. Miller et al. [26] showed that the traditional criteria based on size would incorrectly classify responders as progressive disease on early post 90Y therapy imaging studies for liver metastasis. The measurement of tumor size could be misleading due to hemorrhage and peripheral edema to the treated lesion, which limit the use of RECIST in assessing early response after TARE. EASL and mRECIST criteria were thought to take into account the amount of cellular necrosis using changes in arterial enhancement in the axial plane as a metric of response, which were proposed to measure treatment response to hyper-vascular tumors. All subjects included in our study had hypo-vascular liver metastasis, which are often poorly defined and therefore more difficult to measure accurately due to their minimal rim or perilesional enhancement. In addition, the expected veno-occlusive changes and inflammatory reaction around the targeted lesions after TARE may cause abnormal enhancements which lead to underestimation of the tumor response. Our results are in agreement with a previous study [27] that reported poor correlation between response to LRT achieved by RECIST and EASL for liver metastasis from primary breast cancer. Our study indicates that volumetric ADC, HAE, and PVE can predict the success of the therapy before the conventional axial size measurement in patients with hypo-vascular liver metastases at a functional and molecular level. The combination of different functional MR parameters (ADC, HAE, and PVE) will also help to understand the tumor biology and to better characterize and stage tumors for treatment planning. As a non-invasive biomarkers of early treatment response with hypo-vascular liver metastases, volumetric functional MR metrics can be used to determine whether treatment is effective, which would allow clinicians to optimize individual patient treatment and avoid unnecessary systemic toxicity, expense, and treatment delays.
Our study utilized advanced yet simple and relatively fast software that allows for semi-automated segmentation of the tumor, generating metrics of cellular and molecular changes within the entire tumor volume rather than using ROI measurements in a single axial plain. Tumor necrosis after TARE occurs centrally, and therefore, 3D assessment is more representative of the changes that occur globally within a targeted lesion. Furthermore, the software enabled easy and better reproducibility analysis of multiparametric MR imaging data compared to ROI-based axial measurements [15, 24, 28]. The presented data by Chapiro et al. [29] provided multilayer proof of the precision of 3D quantitative perfusion and diffusion-weighted MR imaging when correlated with pathologic examination as a standard of Ref. [14–16]. Our data demonstrated that volumetric analysis of functional MR imaging during the hepatic arterial and portal venous phase is superior because it takes into account the enhancement of the entire volume of the tumor.
There were several limitations to this study. This is a single-institution retrospective study that included patients with different hypo-vascular metastases who were treated with TARE. This may have resulted in selection bias. Second, our study population was relatively small; and assessing response in each individual tumor type could result in decreasing sample size. Larger sample size study is required to confirm our findings. Third, our study lacks histopathologic correlation of the lesions after 90Y microsphere treatment. Histopathological proof of tumor necrosis after treatment was not feasible nor clinically indicated because of the invasive nature of the procedure, especially in terminal patients with impaired liver function. In the current study, only 2 b values were acquired for DWI. While the acquisition of additional b values may increase the accuracy of ADC calculations, the addition of b values increases sequence acquisition time which could potentially increase the MR imaging exam time. Since quantifying the change in ADC value after therapy rather than the absolute ADC value was the primary objective of this study, and to avoid increase in exam time, we opt to use the 2 b values of 0 and 750 mm2/s. Finally, there may have been some selection bias. Only lesions ≥10 mm were selected for analysis because ADC cannot be accurately measured in smaller lesions.
Conclusions
Quantitative volumetric functional MR imaging can provide an insight into changes at cellular level before tumor size changed, which could be a promising tool in predicting and monitoring the early response to 90Y microsphere treatment of the hypo-vascular liver metastasis as a biomarker. This non-invasive method could benefit the evaluation of early tumor response in future research and clinical trials.
Abbreviations
- TARE:
-
Transarterial radioembolization
- 90Y:
-
Yttrium-90
- RECIST:
-
Response evaluation criteria in solid tumors
- EASL:
-
European association for the study of the liver
- HCC:
-
Hepatocellular carcinoma
- mRECIST:
-
Modified RECIST
- MRI:
-
Magnetic resonance imaging
- DWI:
-
Diffusion-weighted imaging
- ADC:
-
Apparent diffusion coefficient
- LRT:
-
Loco-regional therapy
- NELM:
-
Neuroendocrine liver metastases
- ICLM:
-
Islet cell liver metastases
- TACE:
-
Transarterial chemoembolization
- HAE:
-
Hepatic arterial enhancement
- PVE:
-
Portal venous enhancement
- CR:
-
Complete response
- PR:
-
Partial response
- SD:
-
Stable disease
- PD:
-
Progression of disease
References
Silva AC, Evans JM, McCullough AE, et al. (2009) MR imaging of hypervascular liver masses: a review of current techniques. Radiographics 29(2):385–402
Namasivayam S, Martin DR, Saini S (2007) Imaging of liver metastases: MRI. Cancer Imaging 7:2–9
Cui Y, Zhang XP, Sun YS, Tang L, Shen L (2008) Apparent diffusion coefficient: potential imaging biomarker for prediction and early detection of response to chemotherapy in hepatic metastases. Radiology 248(3):894–900
Halappa VG, Bonekamp S, Corona-Villalobos CP, et al. (2012) Intrahepatic cholangiocarcinoma treated with local-regional therapy: quantitative volumetric apparent diffusion coefficient maps for assessment of tumor response. Radiology 264(1):285–294
Dancey JE, Shepherd FA, Paul K, et al. (2000) Treatment of nonresectable hepatocellular carcinoma with intrahepatic 90Y-microspheres. J Nucl Med 41(10):1673–1681
Jakobs TF, Hoffmann RT, Poepperl G, et al. (2007) Mid-term results in otherwise treatment refractory primary or secondary liver confined tumours treated with selective internal radiation therapy (SIRT) using (90)Yttrium resin-microspheres. Eur Radiol 17(5):1320–1330
Reiner CS, Morsbach F, Sah BR, et al. (2014) Early treatment response evaluation after yttrium-90 radioembolization of liver malignancy with CT perfusion. J Vas Interv Radiol 25(5):747–759
Corona-Villalobos CP, Kamel IR (2014) Functional volumetric MRI in assessing treatment response to intra-arterial therapy of primary and secondary liver tumors. J Comput Assist Tomogr 38(4):513–517
Kamel IR, Reyes DK, Liapi E, Bluemke DA, Geschwind JF (2007) Functional MR imaging assessment of tumor response after 90Y microsphere treatment in patients with unresectable hepatocellular carcinoma. J Vas Interv Radiol 18(1 Pt 1):49–56
Wiering B, Krabbe PF, Jager GJ, Oyen WJ, Ruers TJ (2005) The impact of fluor-18-deoxyglucose-positron emission tomography in the management of colorectal liver metastases. Cancer 104(12):2658–2670
Figueiras RG, Padhani AR, Goh VJ, et al. (2011) Novel oncologic drugs: what they do and how they affect images. Radiographics 31(7):2059–2091
Corona-Villalobos CP, Zhang Y, Zhang WD, Kamel IR (2014) Magnetic resonance imaging of the liver after loco-regional and systemic therapy. Magn Reson Imaging Clin N Am 22(3):353–372
Kamel IR, Bluemke DA, Ramsey D, et al. (2003) Role of diffusion-weighted imaging in estimating tumor necrosis after chemoembolization of hepatocellular carcinoma. AJR Am J Roentgenol 181(3):708–710
Gowdra Halappa V, Corona-Villalobos CP, Bonekamp S, et al. (2013) Neuroendocrine liver metastasis treated by using intraarterial therapy: volumetric functional imaging biomarkers of early tumor response and survival. Radiology 266(2):502–513
Li Z, Bonekamp S, Halappa VG, et al. (2012) Islet cell liver metastases: assessment of volumetric early response with functional MR imaging after transarterial chemoembolization. Radiology 264(1):97–109
Bonekamp S, Li Z, Geschwind JF, et al. (2013) Unresectable hepatocellular carcinoma: MR imaging after intraarterial therapy. Part I. Identification and validation of volumetric functional response criteria. Radiology 268(2):420–430
Vouche M, Kulik L, Atassi R, et al. (2013) Radiological-pathological analysis of WHO, RECIST, EASL, mRECIST and DWI: Imaging analysis from a prospective randomized trial of Y90 ± sorafenib. Hepatology 58(5):1655–1666
Vouche M, Salem R, Lewandowski RJ, Miller FH (2014) Can volumetric ADC measurement help predict response to Y90 radioembolization in HCC? Abdom Imaging. doi:10.1007/s00261-014-0295-6
Grady L (2006) Random walks for image segmentation. IEEE Trans Pattern Anal Mach Intell 28(11):1768–1783
Corona-Villalobos CP, Halappa VG, Bonekamp S, et al. (2015) Functional magnetic resonance imaging response of targeted tumor burden and its impact on survival in patients with hepatocellular carcinoma. Investig Radiol 50(4):283–289
Kamel IR, Bluemke DA (2002) Magnetic resonance imaging of the liver: assessing response to treatment. Top Magn Reson Imaging 13(3):191–200
Assumpcao L, Choti M, Pawlik TM, Gecshwind JF, Kamel IR (2009) Functional MR imaging as a new paradigm for image guidance. Abdom Imaging 34(6):675–685
Llovet JM, Bruix J (2008) Molecular targeted therapies in hepatocellular carcinoma. Hepatology 48(4):1312–1327
Corona-Villalobos CP, Halappa VG, Geschwind JF, et al. (2015) Volumetric assessment of tumour response using functional MR imaging in patients with hepatocellular carcinoma treated with a combination of doxorubicin-eluting beads and sorafenib. Eur Radiol 25(2):380–390
Bonekamp S, Halappa VG, Geschwind JF, et al. (2013) Unresectable hepatocellular carcinoma: MR imaging after intraarterial therapy. Part II. Response stratification using volumetric functional criteria after intraarterial therapy. Radiology 268(2):431–439
Miller FH, Keppke AL, Reddy D, et al. (2007) Response of liver metastases after treatment with yttrium-90 microspheres: role of size, necrosis, and PET. AJR Am J Roentgenol 188(3):776–783
Buijs M, Kamel IR, Vossen JA, et al. (2007) Assessment of metastatic breast cancer response to chemoembolization with contrast agent enhanced and diffusion-weighted MR imaging. J Vasc Interven Radiol 18(8):957–963
Bonekamp D, Bonekamp S, Halappa VG, et al. (2014) Interobserver agreement of semi-automated and manual measurements of functional MRI metrics of treatment response in hepatocellular carcinoma. Eur J Radiol 83(3):487–496
Chapiro J, Wood LD, Lin M, et al. (2014) Radiologic-pathologic analysis of contrast-enhanced and diffusion-weighted MR imaging in patients with HCC after TACE: diagnostic accuracy of 3D quantitative image analysis. Radiology 273(3):746–758
Funding
This study did not receive any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors listed (Xiangyu Zhu, Fatemeh Sobhani, Chunmiao Xu; Li Pan, Mounes Aliyari Ghasebeh, Ihab R. Kamel) have conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
This study was approved by the IRB. Due to the retrospective nature of this study, informed consent was not obtained.
Rights and permissions
About this article
Cite this article
Zhu, X., Sobhani, F., Xu, C. et al. Quantitative volumetric functional MR imaging: an imaging biomarker of early treatment response in hypo-vascular liver metastasis patients after yttrium-90 transarterial radioembolization. Abdom Radiol 41, 1495–1504 (2016). https://doi.org/10.1007/s00261-016-0694-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-016-0694-y