Abstract
Acute traumatic aortic injury (ATAI) is a life-threatening injury. CT is the imaging tool of choice, and the knowledge of direct and indirect signs of injury, grading system, and current management protocol helps the emergency radiologist to better identify and classify the injury and provide additional details that can impact management options. Newer dual-source CT technology with ultrafast acquisition speed has also influenced the appropriate protocol for imaging in patients with suspected ATAI. This review highlights the imaging protocol in patients with blunt trauma, CT appearance and grading systems of ATAI, management options, and the role of the multidisciplinary team in the management of these patients. We also briefly review the current literature on the definition, treatment, and follow-up protocol in patients with minimal aortic injury.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acute traumatic aortic injury (ATAI) is most often a lethal injury with approximately 80% cases with ATAI dying before even reaching the hospital [1, 2]. Among the vehicular crash patients, it is second only to head trauma as the leading cause of posttraumatic deaths [2]. Previously, around 50% patients that reached the hospital with ATAI died within 24 h, but now with better imaging, improving surgical techniques, and percutaneous interventions, the mortality among these patients has decreased to approximately 5% [3]. ATAI occurs in about 2% of cases with blunt thoracic trauma [4]. Approximately 70% of these are automobile vehicle crash victims, while motorcycle, aircraft, pedestrian accidents, fall from height, and crush injury to chest account for the remainder [1]. The exact pathogenesis of ATAI is not clearly understood, but proposed mechanisms include torsion and shearing forces, rapid deceleration, increased intravascular pressure (water-hammer effect), and tearing from the aorta being pinched between the vertebral body and posteriorly displaced sternum (Fig. 1) [5]. Computed tomography angiography (CTA) is the modality of choice for the acute diagnosis of ATAI replacing conventional angiography. CTA enables the assessment of subtle intimal abnormalities and indirect signs of aortic injury such as periaortic hematoma. In the acute setting, electrocardiographic (ECG) gating of CT for aortic imaging [6] is impractical due to the prolonged time for setup, need for additional training of technologists, and longer breath-hold time for the patients. With the advent of newer dual-source CT scanners with ultrafast acquisition speed, acquired images are practically ‘motion-free,’ eliminating the motion artifact seen in the heart and aortic root on conventional CT imaging [7]. With these images in hand, radiological grading of ATAI can be utilized to guide patient management and predict prognosis [8, 9]. This review focuses on up-to-date discussion and recent advances of the imaging protocol, direct and indirect imaging signs, radiological grading of ATAI, definition, and management of minimal aortic injury, and advances in CT imaging that significantly impact the imaging evaluation in patients with trauma.
MDCT Protocol
Due to its diagnostic capability and universal availability, CTA is the investigation of choice for ATAI [5]. As the most common sites for ATAI in patients who present to the hospital are aortic isthmus, ascending aorta, and diaphragmatic hiatus, arterial phase scan of the chest and upper abdomen should be performed in patients with blunt trauma. Most often CTA is included as part of whole-body CT evaluation of polytrauma patients. The advent of dual-source CT scanners and scanners with 64 or more slices has revolutionized the cardiovascular applications of CT [10]. These scanners have the spatial resolution ranging from 0.25 [7] to 0.47 mm, with most newer scanners offering 0.33 mm [10]. These scanners also have a very fast gantry rotation that translates to high temporal resolution [10]. With the new generation of CT scanners, the spatial and temporal resolution continues to increase. Newer dual-energy and 256/320 slice CT machines have allowed coverage of the entire aorta in <3 s [7, 11]. Due to the higher speed of image acquisition, still or motion-free images of even the ascending aorta can be obtained without any ECG gating (Fig. 2). Also, motion artifacts from patient breathing are minimal (also commonly known as ‘free-breathing’ CT [7]), yielding diagnostic quality in almost all cases in our experience. Dual-energy CT scanners can also operate and produce diagnostic images at a lower kV for most patients allowing a lowering of contrast as well as the radiation dose. In our experience, the ability to obtain motion-free non-gated images of the aortic root and the ascending aorta has been the most significant benefit of these newer scanners. In our experience, it is useful to perform arterial phase imaging of chest and upper abdomen followed by portal venous phase acquisition of abdomen and pelvis in a patient with blunt trauma. The reasoning for performing arterial phase imaging of chest and upper abdomen is that most vascular injuries anatomically occur at these sites unless the patient has additional risk factors like a pelvic fracture for pelvic vessel injury. Also, arterial phase-only imaging of chest does not impede detection of any major lung abnormalities; rather, it helps to detect other major cardiovascular injuries [12] that may require specific treatment [13]. A low-dose delayed phase scan of abdomen and pelvis is performed if the injury is confirmed on portal venous phase or if kidney or bladder injury is suspected. Although an initial non-contrast phase is useful for patients with non-traumatic acute aortic emergencies (most useful for diagnosis of intramural hematoma) [6], it is not performed as part of trauma protocol. Chest and abdomen CT protocol for patients with blunt trauma at our institute is highlighted in Table 1. In every case, sagittal and coronal reformats are made from the axial data. Other 3D techniques, including maximum intensity projections (MIP) and volume rendering (VR), are created on a separate workstation. Curved reconstructions of the aorta are created only in cases of preoperative planning for stent placement. These 3D data sets are always read in conjunction with raw data, and these are useful for an overview of vascular anatomy and surgical planning [6, 14].
Axial non-gated CT image at the level of sinus of Valsalva obtained at a newer dual-source scanner (A) (Somatom Definition FLASH, Siemens Medical Solutions, Forchheim, Germany) and a 64-slice MDCT scanner (B) (Somatom Definition AS, Siemens Medical Solutions, Forchheim, Germany) in the same patient. Note the lack of pulsation artifact (arrows) with the newer dual-source scanner even without ECG gating
MRI is almost never used for detection of injury in patients with blunt trauma. However, MR angiogram (MRA) is used for follow-up of injuries, mostly after endovascular stent repair or if renal disease precludes CTA. The MRI protocol for aortic imaging is highlighted in Table 2. If CTA and MRA both cannot be performed due to renal failure (GFR < 30), patients are best followed up by non-contrast CT for interval change in aortic diameter.
Imaging of ATAI
Although CTA will be the definitive modality in most cases, the initial evaluation of trauma often starts with the chest radiograph (CXR). Findings suspicious for ATAI on the CXR include mediastinal widening of >8 cm at the level of aortic arch (or superior mediastinal measuring approximately 25% of the thoracic width), irregularity of the aortic arch, loss of definition of the aortopulmonary window, obscuration of the lung interface with aorta, right side tracheal or nasogastric tube deviation, left main stem bronchus depression, widened left paratracheal stripe, and a left apical cap [5].
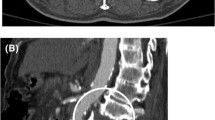
CTA has a very high sensitivity (approximately 98%) and specificity (nearly 100%) for the diagnosis [5]. The CTA signs of ATAI have been divided into direct (or definitive) or indirect (or suggestive). Indirect signs of ATAI include mediastinal or periaortic hematoma, retrocrural hematoma, or a small caliber of the aorta distal to the injury site. The location of a mediastinal hematoma is critical because if there is a distinct fat plane between the aorta and hematoma, alternate sources of bleeding (such as venous, intercostal arterial, or bony) should be considered (Fig. 3). Periaortic hematoma without any definitive sign of aortic injury may be related to an occult intimal injury. Studies have shown that conventional angiography may not provide any additional benefits in patients with occult intimal injury [15], but intravascular ultrasound or transesophageal echocardiography can provide additional information [5]. The direct signs of ATAI include active contrast extravasation, contained rupture (or traumatic pseudoaneurysm), intramural thrombus (IMH), aortic dissection, and abnormality of the aortic contour (including sudden caliber change, also known as pseudocoarctation). When there is a high-risk mechanism of injury such as automobile accident, evaluation of other associated injuries is also critical. The common associations of ATAI are severe head injury, lung and cardiac injury, diaphragmatic rupture, intra-abdominal bleed, pelvis, and long bone fractures [16, 17].
A 36-year-old man with mediastinal hematoma and hemothorax. Oblique sagittal (A) and axial CTA images (B, C) showing well-defined fat plane between the descending aorta and hematoma (arrows), suggesting that aorta is not the source of hematoma. The patient had active bleeding from an intercostal artery (open arrow in C)
Minimal Aortic Injury
The term ‘minimal aortic injury’ (MAI) is an evolving concept that was introduced in 1999 in ‘Presley Trauma Center CT Grading System of Aortic Injury’ (Table 3) by Gavant [18] to describe injuries that are minor in nature. Malhotra et al. [19] described this injury for the first time in patient population and defined it as a small (<1 cm) posttraumatic intimal flap with minimal or no periaortic hematoma. Most of these injuries were undiagnosed by imaging before routine use of CTA. Initially, this injury was estimated to be present in approximately 10% of patients with ATAI [19], but more recent studies have shown that this is seen in approximately 25–35% of cases with ATAI [3, 20–22]. A combination of factors including increased availability of CTA, improvement in CTA technology, and better vehicle safety may be related to increased detection of MAI [23, 24]. The descending aorta is the most common site for MAI, followed by the aortic isthmus [21, 24]. Although the presence of minimal or no mediastinal hematoma is universally accepted, there is no standard definition of aortic abnormality that defines MAI. The varying definition of minimal or minor aortic injury in different studies is highlighted in Table 3. The use of the various definitions of minimal or minor aortic injury is confusing for the emergency radiologists and trauma surgeons as this can affect management decisions. Among these varying definitions, the majority of studies have considered ‘small’ intimal flap, intraluminal thrombus or intramural hematoma without any external contour abnormality and with minimal or no periaortic hematoma as MAI (Figs. 4, 5). Most studies used <1 cm as the definition of ‘small’ as there is higher incidence of disease progression in untreated >1 cm intimal flap or thrombus [3, 8, 19, 20].
A 41-year-old man with motor vehicle collision. Axial CT image of the chest shows a small flap (black arrow) in the anterior descending thoracic aorta without any external hematoma or contour abnormality in keeping with minimal aortic injury. Additional left rib fracture (white arrow) and bilateral pleural effusion are seen
As with the definition of MAI, there is also no consensus on management and follow-up of these injuries. Most studies in the literature have suggested that it can be managed noninvasively with a combination of heart rate, blood pressure control, anticoagulant, or antiplatelet therapy [3, 21, 24, 25]. The selection of appropriate treatment should be done on individual basis, keeping in mind other comorbidities. Initial use of negative inotropes (most commonly beta-blockers) with a goal of systolic BP of 120 mm Hg [26] or mean BP goal of 80 mm Hg [27], and heart rate goal of less than 80 beats per minute [22] to decrease vascular shear stress is suggested. If further supplementation is required, calcium channel blockers or arterial vasodilator are second-line drugs. Although the Society of Vascular Surgery (SVS) guidelines [28] suggest ‘expectant’ management with serial follow-up for intimal injuries, the latest Eastern Association for the Surgery of Trauma (EAST) workgroup management guidelines note that there is insufficient evidence to formulate a recommendation on conservative management of MAI with a need for more research studies [1]. Even the SVS guidelines [28] lack any specific recommendation on ‘expectant’ management and follow-up protocol. The imaging surveillance of MAI usually varies with each particular institution’s experience and their ongoing observations. While the latest study from Harborview Medical Center by Heneghan et al. [3] suggests no need for follow-up imaging, the previous study by the same institute in 2014 by Gunn et al. [21] suggested CTA follow-up at Day 3, 1 week, and 1 month after the injury. Another study by Mosquera et al. [26] suggested a rather long follow-up by CTA at 1, 3, 6, and 12 months after the injury followed by annual cardiovascular magnetic resonance scans. Although there is no consensus on the timeline, most studies suggest that surveillance typically ends when the aorta returns to normal appearance [21, 24, 25]. Almost all studies show that there is no progression of disease in the majority of patients, and if it occurs, it happens in the first month of injury [24, 25]. This disease progression is also supported by experimental animal studies which show that maximal cellular intimal activity and thickening occur 3–4 weeks after the initial insult [29]. Without a consensus or a guideline, it seems reasonable to the authors to obtain surveillance imaging at 1 week and 1 month after injury. If the injury resolves or shows stability or improvement, there may not be a need for further follow-up. Eventually, there is a need for prospective multicenter study using a ‘fixed’ anatomical definition of ‘minor’ aortic injury in which MAI patient group should be consistently managed with predetermined frequency and duration of CTA follow-up.
ATAI Grading
Appropriate classification or grading of ATAI is a topic of an ongoing debate, and several different classification systems have been proposed (Table 4) [3, 9]. One of the most widely used gradings of ATAI was proposed by Azizzadeh et al. [30] (also endorsed by Society of Vascular Surgery [28]) and divides injuries into 4 grades (Table 4): Grade 1—intimal tear (Fig. 5), Grade 2—intramural hematoma or large intimal flap (Fig. 6), Grade 3—pseudoaneurysm (Figs. 7, 8), and Grade 4—free rupture (Fig. 9). Although this grading system provides a straightforward description of the injury, it does not guide treatment [3]. Lamarche et al. [9] proposed Vancouver classification (Table 4). This grading system was reproducible among radiologists, and they showed that the grade correlated well with patient outcome, but does not provide direct grade-based management recommendation. Recently, Heneghan et al. [3] proposed ‘Harborview Classification’ (Table 4) of ATAI that also helps to guide management based on the grade of injury. Harborview classification divides ATAI into minimal, moderate, and severe injury and suggests that patients should be managed by follow-up imaging, initial stabilization of concomitant injuries, and urgent repair of aortic injury, respectively. In view of authors, currently, the Vancouver classification may be the most appropriate classification to use as it has less interobserver variability and a few studies have validated its correlation with outcomes [9, 31]. Multicenter studies focusing on the classification systems, their outcomes, and ability to guide treatment may help to establish a standard grading system in future.
A 45-year-old man with blunt trauma. Axial (A), coronal (B), and sagittal (C) CTA images reveal acute traumatic aortic injury in the proximal descending aorta causing intramural hematoma in aortic arch, arch vessels (curved arrow in C) and the descending thoracic aorta (white arrows) with a thrombus (open arrows in A and C). Fractures of the sternal manubrium and left ribs were also present (not shown)
A 33-year-old man with traumatic thoracic injury. Axial (A) and coronal (B) ECG-gated CTA images reveal pseudoaneurysm of the ascending thoracic aorta from its medial left wall, just above the sinotubular junction. It measured 1.4 × 0.7 cm with a neck of 1 cm. No evidence of active contrast extravasation. Mild mediastinal fat stranding related to small mediastinal hemorrhage (open arrow). These findings are important to note for treatment planning
Treatment Planning of ATAI
The clinician caring for a patient with ATAI has a variety of options available to treat the injury including traditional open repair, endovascular stenting, and an increasing focus on conservative management in appropriately selected patients [3, 24, 30]. Thus, CTA grading of ATAI is critical for the treatment planning of ATAI. In addition to the information on severity and type of injury, CTA reports should include aortic arch anatomy, vertebral artery dominance, any significant preexistent atherosclerotic disease or stenosis, prior postoperative changes (like CABG), length of aortic injury, aortic diameter above and below the injury, and proximity to the origin of left subclavian artery (in case of isthmic injuries). This information aids the clinician in deciding between the treatment options and also guides the intervention. For example, surgical aortic repair usually involves a left posterolateral thoracotomy often requiring cardiopulmonary bypass. Imaging helps a surgeon to identify the exact area of injury and to assess any branch vessel involvement, and it also provides information on other injuries that can be treated in the same setting. It also helps to decide the anatomical landmark to place the clamp or bypass cannula [5]. Few additional important radiological findings to highlight in patients being planned for endovascular stent include the presence of any focal lesion or thrombus, information on proximal and distal landing zone (preferably >2 cm length), the presence of calcification in the landing zone, tortuosity of the aorta, and diameter of access vessels [6, 32].
Management of ATAI
There is a debate among the best management option for these patients. Among the patients that need procedural repair, studies have shown that the increasing utilization of endovascular stents has led to a decreased rate of early complications and reduced hospital stay [1, 3, 16, 26, 30]. The latest EAST guidelines [1] recommend the use of endovascular repair in patients that do not have contraindications to the procedure. The guidelines also suggest that in hemodynamically stable patients, the definitive management of ATAI should be delayed until other acute life-threatening injuries are adequately managed and appropriately resuscitated. An early management of ATAI may be associated with higher incidence of paraplegia and renal failure [1]. But if the patient is hemodynamically unstable and there are direct signs of the aortic injury on CT, definitive emergent management of the aortic injury is suggested. The suggested management algorithm of ATAI in patients with blunt trauma based on recommendations of EAST Practice Work Group recommendations is highlighted in Table 5.
Trends in Management and Follow-Up
Analysis of the National inpatient sample by Ultee et al. [33] revealed that management by TEVAR has increased from 6.5% of the operative volume in 2005 to 87% in 2011. The overall mortality for aortic trauma has also shown a decrease, this being attributed to the use of TEVAR, broadening of indications of surgical repair, and other technical improvements. Aortic trauma being relatively common in a younger age group brings in many unique problems with respect to its treatment and outcomes. The average aortic size in this population is much smaller than aneurysmal disease, and the early experience with EVAR thus faced problems with graft sizing. The geometry of the arch in younger patients is also more pointed, and this is associated with a poor conformability of the endograft to the aortic arch. A 0–18% rate of infolding, migration, stent graft collapse, and even acute aortic occlusion requiring re-intervention is reported, with a majority of the events occurring early. Aortic remodeling in these patients may also differ with the aorta just distal to the left subclavian artery expanding at a greater rate than the proximal segments [34, 35]. Other than technical advances in the devices, utilization of procedural modifications [36] such as limiting the graft oversizing to 10% to accommodate for this subsequent remodeling have been suggested. The use of off-label devices, FEVAR, BEVAR, chimney, snorkel is all reported, but the overall numbers are too small for robust conclusions [37]. The effect of these factors on the long-term outcomes remains an area that requires further study.
With hybrid operating rooms and modern angio-suites becoming more common, parallel improvements in imaging like cone-beam CT, fusion imaging, and IVUS have also facilitated the operative and interventional procedure, minimizing irradiation and contrast usage [38]. This as well as the better image quality is crucial for the technical success of complex aortic endovascular procedures. Better planning software and fusion imaging are useful for accurate graft deployment, and this can be further confirmed by IVUS. Utilization of IVUS in aortic interventions improves understanding of complex anatomy and is shown to reduce the amount of contrast utilized and overall operating room time [39, 40]. The use of the cone-beam CT at the end of the procedure enables detection of structural problems that can then be tackled immediately, reducing re-intervention and morbidity [41]. Overall, there are many ethical and personnel issues involved in the management of these patients as newer treatment technology becomes available. Lifesaving salvage interventions take priority with planned second-look angio-suite or operating room visits. Depending on available facilities, centers may decide to proceed with intervention in an angio-suite, but should have the other teams involved in care on alert, in case the patient needs to be taken in for surgery. In centers with modern angio-suites, treatment of a patient with isolated aortic trauma may be feasible away from operating rooms [42].
Follow-up of the patients undergoing endovascular stent repair is a matter of debate, and a universal protocol has not been defined. Graft surveillance is needed as aorta dilates at the site of implantation and the dilation is more in patients that receive a stent for traumatic injury repair than for aneurysm repair [43]. Mostly, CTA is done for follow-up, but since traumatic injuries are more common in the relatively younger population, there is an increasing interest in the use of MRI (as most of the approved stent grafts are MRI compatible) for surveillance due to cumulative radiation exposure. The MRI protocol is highlighted in Table 2. Many centers do surveillance by CTA that are performed at 1, 6 months, then annually for first 5 years, and more liberally thereafter (mostly every 2–3 years) on case-to-case basis [35, 44]. An analysis of long-term outcomes is difficult due to the confounding effect of other associated injuries and a significant number of patients being lost to follow-up. In our experience, CTA should be used for initial examinations, and MRI can be utilized for subsequent follow-up examinations. When MRI is performed, it may be useful to perform a radiograph (most commonly chest in orthogonal views) as they can also effectively diagnose stent migration, kinking, and deformity [45].
Multidisciplinary Approach to Management
The patient with blunt trauma requires an interdisciplinary management approach. At our institution, we have developed multispecialty teams for rapid assessment, triage, and management of patients with suspected acute aortic emergencies (based on physical examination, external or presentation imaging, or known prior history of aortic disease). This team includes specialists in cardiovascular imaging, interventional radiology, cardiac surgery, vascular and endovascular surgery, cardiology, vascular medicine, and cardiac and vascular anesthesia. The team collaborates to provide expert evaluation for all acute aortic emergencies and discuss multiple approaches to treatment, including endovascular techniques, minimally invasive cardiac procedures, and open procedures, for patients with aortic disease. In our experience, this multidisciplinary team approach for these patients is crucial to triage and manage them appropriately.
Conclusion
Recent advances in CTA help in making a rapid and accurate diagnosis of aortic injuries in the emergency department. The knowledge of direct and indirect imaging signs is quintessential for acute care radiologists. Incorporating the newer concepts of traumatic grading and minimal aortic injury into day-to-day practice will help in further guiding the management of our trauma surgeons. Finally, a multidisciplinary team approach is required for managing the life-threatening condition of ATAI to improve patient outcomes.
References
Fox N, Schwartz D, Salazar JH, et al. Evaluation and management of blunt traumatic aortic injury: a practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2015;78(1):136–46.
Neschis DG, Scalea TM, Flinn WR, Griffith BP. Blunt aortic injury. N Engl J Med. 2008;359(16):1708–16.
Heneghan RE, Aarabi S, Quiroga E, Gunn ML, Singh N, Starnes BW. Call for a new classification system and treatment strategy in blunt aortic injury. J Vasc Surg. 2016;64(1):171–6.
Ungar TC, Wolf SJ, Haukoos JS, Dyer DS, Moore EE. Derivation of a clinical decision rule to exclude thoracic aortic imaging in patients with blunt chest trauma after motor vehicle collisions. J Trauma. 2006;61(5):1150–5.
Steenburg SD, Ravenel JG, Ikonomidis JS, Schonholz C, Reeves S. Acute traumatic aortic injury: imaging evaluation and management. Radiology. 2008;248(3):748–62.
Nagpal P, Khandelwal A, Saboo SS, Bathla G, Steigner ML, Rybicki FJ. Modern imaging techniques: applications in the management of acute aortic pathologies. Postgrad Med J. 1078;2015(91):449–62.
Seimens Healthineers USA. http://static.healthcare.siemens.com/siemens_hwem-hwem_ssxa_websites-context-root/wcm/idc/groups/public/@us/@imaging/documents/download/mdaz/nzi5/~edisp/force_brochure_4_2014-01645832.pdf. Accessed July 16 2016.
Starnes BW, Lundgren RS, Gunn M, et al. A new classification scheme for treating blunt aortic injury. J Vasc Surg. 2012;55(1):47–54.
Lamarche Y, Berger FH, Nicolaou S, et al. Vancouver simplified grading system with computed tomographic angiography for blunt aortic injury. J Thorac Cardiovasc Surg. 2012;144(2):347–54.
Rogalla P, Kloeters C, Hein PA. CT technology overview: 64-slice and beyond. Radiol Clin North Am. 2009;47(1):1–11.
Voitle E, Hofmann W, Cejna M. Aortic emergencies-diagnosis and treatment: a pictorial review. Insights Imaging. 2015;6(1):17–32.
Nagpal P, Saboo SS, Khandelwal A, Duran-Mendicuti MA, Abbara S, Steigner ML. Traumatic right atrial pseudoaneurysm. Cardiovasc Diagn Ther. 2015;5(2):141–4.
Soto JA, Anderson SW. Multidetector CT of blunt abdominal trauma. Radiology. 2012;265(3):678–93.
Fishman EK, Ney DR, Heath DG, Corl FM, Horton KM, Johnson PT. Volume rendering versus maximum intensity projection in CT angiography: what works best, when, and why. Radiographics. 2006;26(3):905–22.
Sammer M, Wang E, Blackmore CC, Burdick TR, Hollingworth W. Indeterminate CT angiography in blunt thoracic trauma: is CT angiography enough? AJR Am J Roentgenol. 2007;189(3):603–8.
Fabian TC, Richardson JD, Croce MA, et al. Prospective study of blunt aortic injury: Multicenter Trial of the American Association for the Surgery of Trauma. J Trauma. 1997;42(3):374–80 (discussion 80–83).
Kram HB, Appel PL, Wohlmuth DA, Shoemaker WC. Diagnosis of traumatic thoracic aortic rupture: a 10-year retrospective analysis. Ann Thorac Surg. 1989;47(2):282–6.
Gavant ML. Helical CT grading of traumatic aortic injuries. Impact on clinical guidelines for medical and surgical management. Radiol Clin North Am. 1999;37(3):553–74.
Malhotra AK, Fabian TC, Croce MA, Weiman DS, Gavant ML, Pate JW. Minimal aortic injury: a lesion associated with advancing diagnostic techniques. J Trauma. 2001;51(6):1042–8.
Forman MJ, Mirvis SE, Hollander DS. Blunt thoracic aortic injuries: CT characterisation and treatment outcomes of minor injury. Eur Radiol. 2013;23(11):2988–95.
Gunn ML, Lehnert BE, Lungren RS, et al. Minimal aortic injury of the thoracic aorta: imaging appearances and outcome. Emerg Radiol. 2014;21(3):227–33.
Paul JS, Neideen T, Tutton S, et al. Minimal aortic injury after blunt trauma: selective nonoperative management is safe. J Trauma. 2011;71(6):1519–23.
de Mestral C, Dueck A, Sharma SS, et al. Evolution of the incidence, management, and mortality of blunt thoracic aortic injury: a population-based analysis. J Am Coll Surg. 2013;216(6):1110–5.
Osgood MJ, Heck JM, Rellinger EJ, et al. Natural history of grade I–II blunt traumatic aortic injury. J Vasc Surg. 2014;59(2):334–41.
Kidane B, Abramowitz D, Harris JR, DeRose G, Forbes TL. Natural history of minimal aortic injury following blunt thoracic aortic trauma. Can J Surg Journal canadien de chirurgie. 2012;55(6):377–81.
Mosquera VX, Marini M, Lopez-Perez JM, et al. Role of conservative management in traumatic aortic injury: comparison of long-term results of conservative, surgical, and endovascular treatment. J Thorac Cardiovasc Surg. 2011;142(3):614–21.
Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35(41):2873–926.
Lee WA, Matsumura JS, Mitchell RS, et al. Endovascular repair of traumatic thoracic aortic injury: clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg. 2011;53(1):187–92.
Neville RF, Padberg FT Jr, DeFouw D, Hernandez J, Duran W, Hobson RW 2nd. The arterial wall response to intimal injury in an experimental model. Ann Vasc Surg. 1992;6(1):50–4.
Azizzadeh A, Keyhani K, Miller CC 3rd, Coogan SM, Safi HJ, Estrera AL. Blunt traumatic aortic injury: initial experience with endovascular repair. J Vasc Surg. 2009;49(6):1403–8.
Forcillo J, Philie M, Ojanguren A, et al. Outcomes of traumatic aortic injury in a primary open surgical approach paradigm. Trauma Mon. 2015;20(2):e18198.
Fanelli F, Dake MD. Standard of practice for the endovascular treatment of thoracic aortic aneurysms and type B dissections. Cardiovasc Intervent Radiol. 2009;32(5):849–60.
Ultee KH, Soden PA, Chien V, et al. National trends in utilization and outcome of thoracic endovascular aortic repair for traumatic thoracic aortic injuries. J Vasc Surg. 2016;63(5):1232–9.
Forbes TL, Harris JR, Lawlor DK, Derose G. Aortic dilatation after endovascular repair of blunt traumatic thoracic aortic injuries. J Vasc Surg. 2010;52(1):45–8.
Farber MA, Mendes RR. Endovascular repair of blunt thoracic aortic injury: techniques and tips. J Vasc Surg. 2009;50(3):683–6.
Kolbel T, Dias N, Resch T, Holst J, Sonesson B, Malina M. In situ bending of thoracic stent grafts: clinical application of a novel technique to improve conformance to the aortic arch. J Vasc Surg. 2009;49(6):1613–6.
Neschis DG, Moainie S, Flinn WR, Scalea TM, Bartlett ST, Griffith BP. Endograft repair of traumatic aortic injury—a technique in evolution: a single institution’s experience. Ann Surg. 2009;250(3):377–82.
Stangenberg L, Shuja F, Carelsen B, Elenbaas T, Wyers MC, Schermerhorn ML. A novel tool for three-dimensional roadmapping reduces radiation exposure and contrast agent dose in complex endovascular interventions. J Vasc Surg. 2015;62(2):448–55.
Song TK, Donayre CE, Kopchok GE, White RA. Intravascular ultrasound use in the treatment of thoracoabdominal dissections, aneurysms, and transections. Semin Vasc Surg. 2006;19(3):145–9.
Guo BL, Shi ZY, Guo DQ, et al. Effect of intravascular ultrasound-assisted thoracic endovascular aortic repair for “complicated” type B aortic dissection. Chin Med J (Engl). 2015;128(17):2322–9.
Dijkstra ML, Eagleton MJ, Greenberg RK, Mastracci T, Hernandez A. Intraoperative C-arm cone-beam computed tomography in fenestrated/branched aortic endografting. J Vasc Surg. 2011;53(3):583–90.
Bashore TM, Bates ER, Berger PB, et al. American College of Cardiology/Society for Cardiac Angiography and Interventions Clinical Expert Consensus Document on cardiac catheterization laboratory standards. A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001;37(8):2170–214.
Alberta HB, Secor JL, Smits TC, et al. Comparison of thoracic aortic diameter changes after endograft placement in patients with traumatic and aneurysmal disease. J Vasc Surg. 2014;59(5):1241–6.
Watson J, Slaiby J, Garcia Toca M, Marcaccio EJ Jr, Chong TT. A 14-year experience with blunt thoracic aortic injury. J Vasc Surg. 2013;58(2):380–5.
Fearn S, Lawrence-Brown MM, Semmens JB, Hartley D. Follow-up after endovascular aortic aneurysm repair: the plain radiograph has an essential role in surveillance. J Endovasc Ther. 2003;10(5):894–901.
Riesenman PJ, Brooks JD, Farber MA. Acute blunt traumatic injury to the descending thoracic aorta. J Vasc Surg. 2012;56(5):1274–80.
Estrera AL, Miller CC 3rd, Guajardo-Salinas G, et al. Update on blunt thoracic aortic injury: fifteen-year single-institution experience. J Thorac Cardiovasc Surg. 2013;145(3 Suppl):S154–8.
Rabin J, DuBose J, Sliker CW, O’Connor JV, Scalea TM, Griffith BP. Parameters for successful nonoperative management of traumatic aortic injury. J Thorac Cardiovasc Surg. 2014;147(1):143–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All of the authors report no relationships that could be construed as a conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Does not apply.
Rights and permissions
About this article
Cite this article
Nagpal, P., Mullan, B.F., Sen, I. et al. Advances in Imaging and Management Trends of Traumatic Aortic Injuries. Cardiovasc Intervent Radiol 40, 643–654 (2017). https://doi.org/10.1007/s00270-017-1572-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-017-1572-x