Abstract
Purpose of Review
This article aims to review the key aspects of the imaging evaluation of acute traumatic aortic injury (ATAI) with an emphasis on factors that affect management of these patients.
Recent Findings
In the setting of trauma, the chest radiograph typically serves as the initial imaging evaluation and can be useful in detecting signs of mediastinal hematoma. In the current era, definitive diagnosis of ATAI is made with computed tomography (CT), where indirect and direct findings of ATAI can enable a confident diagnosis. Knowledge of potential technical and anatomic CT imaging pitfalls can prevent misdiagnosis of ATAI.
Summary
Diagnosis of ATAI in the setting of blunt or penetrating trauma relies heavily on timely and accurate imaging interpretation. Once the diagnosis is made, a meaningful report including appropriate descriptors of the characteristics and location of ATAI should be generated by the radiologist to help direct management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acute traumatic aortic injury (ATAI) occurs in the setting of blunt or penetrating trauma and may result in adverse outcomes. While the diagnosis of ATAI can be confidently made on computed tomography (CT), mimics of ATAI are common in this patient population which often has other life-threatening injuries. Accurate and timely interpretation is key to ensure that patients with ATAI are treated immediately, and those without ATATI do not undergo additional or unnecessary procedures.
Blunt trauma accounts for the majority of ATAI with approximately 75–80% the result of high-speed motor vehicle collisions [1]. Up to 70% of these injuries are lethal at the time of the accident [1, 2]. Although the exact mechanism of aortic injury in the setting of blunt trauma is not known, it is presumed to involve shearing forces caused by rapid differential deceleration [1]. The most common location for imaged ATAI in the setting of blunt trauma is at the aortic isthmus (~ 90%), just distal to the origin of the left subclavian artery, due to the relatively fixed position of the aorta at the site of the ligamentum arteriosum [3]. Penetrating trauma to the thorax in the setting of gunshot or knife wounds can also result in direct injury to the aorta. These injuries are directly related to the trajectory of the weapon.
Early diagnosis of ATAI is important, as up to 90% of patients arriving at the hospital will survive repair [4]. The clinical presentation of ATAI is variable, and depends on the mechanism and magnitude of injury. Patients can present with clinical symptoms that are neither sensitive nor specific, including chest pain, back pain, and difficulty breathing. The clinical signs of ATAI, including external chest wall injuries, systemic hypotension, concomitant upper limb hypertension, or difference in blood pressures between the right and left brachial arteries, may be absent in up to one-third of patients [5]. Adding to the confusion, some patients may initially present without clinical signs or symptoms and rapidly develop hemodynamic instability secondary to an underlying ATAI.
Given potentially fatal outcomes and the non-specific clinical signs and symptoms of ATAI, emphasis is placed on rapid thoracic imaging in the initial assessment of both blunt and penetrating trauma. Imaging findings seen on the initial chest radiograph when combined with clinical assessment may raise the suspicion of potential ATAI and prompt the need for CT imaging of the aorta. The radiologist plays an integral role in diagnosis of ATAI, directing further management guided by the severity of injury demonstrated on CT. The purpose of this article is to review the imaging findings of ATAI and summarize the factors guiding treatment of patients with ATAI.
Imaging of ATAI: Chest Radiograph
The chest radiograph is typically the first diagnostic imaging examination that trauma patients undergo upon presenting to the emergency department. The primary purpose of the chest radiograph is to evaluate positioning of support lines and determine if potentially life-threatening injuries that require immediate treatment, such as tension pneumothorax or large hemothorax, are present. The radiograph may also demonstrate findings suggestive of a mediastinal hematoma, which requires further investigation for ATAI.
Initial evaluation for mediastinal hematoma on the chest radiograph focuses on assessment of the width of the mediastinum (Fig. 1). Mediastinal widening is defined as a width greater than 6 cm on an upright posteroanterior chest radiograph (or 8 cm on a supine anteroposterior chest radiograph) of at least 25% of the thoracic width at the level of the left subclavian artery [6]. Mediastinal widening is a fairly sensitive indicator for mediastinal hematoma; however, it is not very specific as there are several other causes of mediastinal widening including mediastinal lipomatosis, vascular anomalies, lymphadenopathy, or technique-related factors [5, 7].
Portable supine anterosuperior chest radiograph (a) demonstrates widening of the mediastinum (arrows), highly suspicious for mediastinal hematoma in this patient status post motor vehicle collision. Coronal reconstruction of a chest CT with intravenous contrast (b) confirms the presence of a periaortic, mediastinal hematoma (asterisks) with a focal outpouching arising from the aortic isthmus (arrow), indicative of acute traumatic aortic injury
Other radiographic signs should be used in addition to widening in the mediastinal assessment to improve accuracy, including indistinctness of the aortic knob or descending thoracic aorta (Fig. 2). Normally, the transverse and descending aortic contour should have a crisp and clear interface with the adjacent lung and aortopulmonary window. Obscuration of the aortic contour should raise suspicion for an adjacent hematoma and potential aortic injury. Depression of the left mainstem bronchus, rightward deviation of the trachea, or deviation of support devices including endotracheal and enteric tubes should also raise suspicion of mass effect from hematoma due to possible ATAI (Fig. 3). Left apical capping (defined as extrapleural blood overlying the left apex) is a finding occasionally seen in the setting of ATAI and is due to hemorrhage tracking along the reflection of the left subclavian artery [5] (Fig. 4). Other radiographic findings include displacement of the left paravertebral stripe and the presence of an additional vertical interface bisecting the aortic knob. Since most imaged ATAI involves the aortic isthmus, imaging features tend to consist of widening of the left paratracheal region with mass effect on adjacent structures displacing them away from the isthmus. Isolated-right-sided mediastinal widening is typically the result of brachiocephalic or esophageal injury, rather than aortic injury. Isolated superior mediastinal widening may also be seen in great vessel injury.
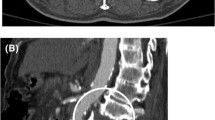
Initial supine anteroposterior chest radiograph (a) in this patient following high-speed motor vehicle collision demonstrates rightward deviation of the nasogastric tube (arrows) with an accompanying enlargement and abnormal contour of the aortic knob (asterisk), concerning for aortic injury. There are also multiple right-sided rib fractures (ellipse) as well as a right pneumothorax and subcutaneous emphysema within the right chest wall (arrowhead). Transaxial chest CT with intravenous contrast image (b) demonstrates mediastinal hemorrhage with active extravasation of contrast from the proximal descending aorta (arrow), a direct sign of traumatic aortic injury
Portable anteroposterior chest radiograph (a) demonstrates mediastinal widening with asymmetric left apical capping (ellipse), suspicious for vascular injury in this patient following blunt chest trauma. Coronal reconstruction of a chest CT with intravenous contrast (b) demonstrates hemorrhage tracking within the left superomedial extrapleural space (circle). Inferiorly at the level of the aortic isthmus (c), there is an intraluminal tissue flap (arrow), confirming aortic injury
At our institution, in the setting of trauma, any one of these suspicious findings on chest radiograph merits further imaging. In patients with a low clinical suspicion for ATAI, an upright posteroanterior chest radiograph can be obtained, as mediastinal widening seen on supine anteroposterior radiographs may resolve with upright positioning [5]. However, in 2018, any persistent concern based on clinical or chest radiograph findings should be further evaluated with CT. In our algorithm, chest radiography rarely remains the only thoracic study when the mechanism of injury is sufficient, the patient is unable to verbalize the presence of chest pain, or when other serious injuries exist that may serve as distracting factors.
Imaging of ATAI: CT
CT is the modality of choice in diagnosing ATAI, with a sensitivity greater than 96% and specificity of greater than 99% [1, 7]. Intravenous contrast is essential to delineate the vasculature and assess associated findings. At our institution, we obtain CT images in a single phase with a fixed delay at 70 s with the arms raised above the patient’s head. Images are obtained in a single pass from the thoracic inlet through femoral heads using a standard 3-mm slice thickness reconstructed at 2-mm intervals with the option to reconstruct thinner slices. We prefer to use a fixed delay at 70 s (similar to a portal venous phase), rather than an arterial phase (CT angiography), as this allows for one continuous scan of the chest, abdomen, and pelvis to evaluate for both aortic and visceral injury with high accuracy. A review of 281 patients demonstrated no difference in CT angiography vs. routine portal venous phase chest CT in diagnosing ATAI [8]. Routine use of multiplanar reconstructions (MPR) allows for a more detailed evaluation of the aorta and may detect more subtle findings of ATAI.
Evaluation for ATAI on CT requires detection of indirect and direct signs of vascular injury. The indirect sign of vascular injury on CT is the presence of adjacent hematoma, which is usually caused by avulsion of perivascular veins from the arterial vasa vasorum [6], rather than extravasation of intraluminal blood from the vessel itself. A mediastinal hematoma presents as an area of hyperattenuating material (typically 40–70 HU) that effaces the fat plane with the involved vessel and displaces and infiltrates normal mediastinal fat. To suggest underlying ATAI, careful attention must be made to identify that the hematoma is in direct contact with the aorta with loss of a separating fat plane (Fig. 5). Other traumatic etiologies, such as a venous bleed or fracture, can similarly produce mediastinal hemorrhage, thus decreasing the specificity for ATAI of a mediastinal hematoma detected without direct signs of ATAI (Fig. 6) [9,10,11]. It is also important to recognize that the presence of a periaortic mediastinal hematoma is not a requirement for ATAI, as ATAI may rarely occur in the absence of hematoma [12, 13].
Transaxial (a) and coronal reconstruction (b) chest CT with intravenous contrast images demonstrate a large mediastinal hematoma in contact with the thoracic aorta with loss of the separating fat plane (asterisks). Direct evidence of aortic injury is also present as evidenced by an adjacent, irregular intraluminal tissue flap (circle)
Transaxial chest CT with intravenous contrast image demonstrates an anterior mediastinal hematoma (arrow) with a preserved fat plane (dashed ellipse) between the hematoma and aorta. No direct signs of traumatic aortic injury were identified, and the hematoma was felt to be secondary to venous injury
Direct signs of ATAI represent changes of the imaging appearance of the aorta itself. There are numerous direct signs of ATAI on CT indicating varying levels of injury severity [5]. The most basic direct finding of ATAI on CT is disruption of the aortic wall which can manifest as either a focal outpouching containing contrast (Fig. 7), a contour irregularity in the aortic wall, or in rare cases, extravasation of contrast from the lumen of the aorta (Fig. 8). Although the term “pseudoaneurysm” is frequently reported in the literature, we prefer to use the term focal outpouching as the vessel may be ruptured or transected and pseudoaneurysm may underestimate the degree of injury or imply some sense of stability.
Transaxial chest CT with intravenous contrast image depicts a focal outpouching of contrast at the level of the aortic isthmus (arrow), a direct sign of traumatic injury, with periaortic hemorrhage. We prefer to use the term “focal outpouching” rather than “pseudoaneurysm,” as the latter may inadvertently underestimate the degree of severity
In some cases of ATAI, the contour of the aorta may be preserved; however, there will be findings to suggest injury to the intima of the aorta. Direct signs of injury to the intima of the aorta include eccentric thrombus and strands of tissue within the lumen. Strands of tissue or tissue flaps within the lumen of the aorta are a direct finding of aortic injury (Fig. 9). Although often reported in the literature as “dissection flap,” we prefer to avoid use of this term as “dissection” implies an intimomedial flap, and, in the setting of ATAI, injury may be to the adventia, intima, media, or multiple layers of the wall of the aorta. ATAI involving the intima alone can also result in thrombus formation and serve as a nidus for distal embolic infarctions (Fig. 10).
Transaxial chest CT with intravenous contrast image demonstrates a tissue flap across the proximal descending thoracic aorta (arrows) with surrounding hemorrhage. We prefer avoid using the term “dissection flap,” as this implies injury isolated injury to the media when, in fact, in cases of aortic trauma, injury may extend to additional layers of the aortic wall
High attenuation within the aortic wall is a rarer but important direct sign of ATAI, indicative of an underlying intramural hematoma (Fig. 11). A change in aortic caliber can also be seen and may be due to intramural hematoma or external compression of the aorta itself by adjacent mediastinal hemorrhage (Fig. 12).
Transaxial chest CT with intravenous contrast image depicts high-attenuating thickening of the aortic wall (asterisks) which, in the setting of trauma, is consistent with intramural hematoma, another direct sign of traumatic aortic injury. This finding resolved on a follow-up examination 48 h later, confirming that it is related to injury and not atherosclerotic disease. In the absence of a non-contrast study or prior examinations for comparison, this finding may be difficult to perceive, particularly in the setting of underlying atherosclerotic disease
Oblique sagittal reconstructed chest CT with intravenous contrast image demonstrates long-segment narrowing of the descending thoracic aorta, secondary to extrinsic compression from an adjacent posterior mediastinal hematoma (asterisks). There is also extensive active contrast extravasation from the aorta into this hematoma (arrow), indicative of life-threatening aortic rupture
Penetrating aortic injury is much less commonly seen, however, warrants mention, as the indirect and direct signs described above may be absent in some cases. Trajectory of the offending weapon serves as an indirect sign of injury (Fig. 13). Any path that traverses or passes near the aorta should be suspicious for ATAI, particularly in the presence of a mediastinal hematoma that effaces the fat plane with the aorta. A penetrating bullet wound can also result in surrounding blast injury to the aorta without directly passing through the vessel. In all cases of penetrating injury, the projectile or weapon tract should be traced with multiplanar reconstructions to better delineate potential sites of injury. In our practice, in the setting of penetrating injury, the presence of a mediastinal periaortic hematoma without direct signs of ATAI is typically managed conservatively with follow-up CT in 48–72 h to document stability [5].
Supine anteroposterior chest radiograph (a) in this patient following a gunshot wound to the back demonstrates multiple bullet fragments overlying the thorax along with near complete opacification of the left hemithorax and loss of the normal aortic contour (arrows), worrisome for aortic injury. Transaxial (b) and oblique sagittal reformatted (c) chest CT with intravenous contrast images shows bullet fragments within the posterior mediastinum and spine (ellipses) and multiple areas of active extravasation (solid arrows) within a large periaortic hematoma along the trajectory of the bullets (dashed arrow), confirming penetrating traumatic aortic injury
Abdominal acute traumatic aortic injuries are much less common than those in the chest but are becoming increasingly recognized and are strongly associated with vertebral body fractures [14]. The same principles of indirect and direct signs of thoracic aortic injury apply within the abdomen and pelvis, and can be used in concert with associated visceral injuries (Fig. 14).
Transaxial abdominal CT with intravenous contrast image (a) demonstrates a large retroperitoneal hematoma surrounding the abdominal aorta (asterisks) in this patient status post gunshot wound to the lower back. Slightly more inferior (b), multiple bullet fragments are noted in the spine and abdomen with numerous foci of active contrast extravasation (arrows). Sagittal reconstruction image (c) shows the areas of active extravasation (solid arrows) along the trajectory of the bullets (dashed arrow) with complete non-visualization of the abdominal aorta below the plane of injury. The patient underwent emergent repair with aortobifemoral bypass grafting
CT Mimics and Pitfalls
There are several factors which may make accurate interpretation and diagnosis of ATAI challenging. Technique-related CT artifacts, including streak, flow, respiratory motion, and vascular pulsation, may either simulate or hide underlying ATAI [5]. Streak artifact overlying the aorta is most commonly the result of dense contrast within the superior vena cava (Fig. 15). Using a saline chaser following contrast injection or a longer delay in image acquisition can help mitigate streak artifact. Objects outside the thorax, most commonly the patient’s arms, can result in photon deprivation and inadequate visualization of the aorta, and can be prevented by careful attention to patient positioning with the arms raised above the head prior to scanning. Flow-related phenomena are visualized as ill-defined, smoke-like intraluminal filling defects, and are often seen in patients with decreased cardiac output who are imaged with fast, high-pitch scanning techniques (Fig. 16). If recognized at the scanner, an immediate repeat examination will allow for full opacification of the aorta, thereby yielding a diagnostic study. Respiratory motion may simulate various direct signs of ATAI and can be confirmed by assessing the aorta in multiple planes and evaluating lung windows. Vascular pulsation artifact is noticeably worse in the ascending aorta due to cardiac motion (Fig. 17). When encountered, assess for the presence of simultaneous artifact in the main pulmonary artery and consider repeat examination with ECG-gating or fast, high-pitch techniques.
Transaxial chest CT with intravenous contrast image reveals an ill-defined, smoke-like intraluminal filling defect within the ascending aorta (arrow). Given the lack of surrounding hematoma and other evidence to suggest traumatic aortic injury, this was secondary to flow artifact in the setting of low cardiac output
Transaxial chest CT with intravenous contrast (a) performed at an outside institution in a patient transferred following a high-speed motor vehicle collision demonstrates poor visualization of the wall of the ascending aorta with multiple curvilinear densities extending outward, overlapping the aorta and right ventricular outflow tract (arrows). Although these findings were felt to represent vascular pulsation artifact, traumatic aortic injury could not be definitively excluded. An ECG-gated CT of the chest (b) was subsequently performed, demonstrating a normal-appearing aorta without evidence of traumatic injury
Normal anatomic structures and variants can also simulate signs of ATAI. Residual thymic tissue within the anterior mediastinum can mimic a hematoma. However, unlike a mediastinal hematoma, which is ill-defined and insinuates into the adjacent fat, thymic tissue is typically triangular-shaped and is not accompanied by surrounding fat stranding. Similarly, pericardial recesses adjacent to the aorta can be confused with mediastinal or intramural hematoma. Near water attenuation and the preservation of a fat plane between the recess and the aorta help to differentiate this finding from a mediastinal hematoma. The ductus diverticulum is a normal anatomic variant located along the undersurface of the aorta at the isthmus (the most common site for aortic injury). Unlike ATAI, which appears more irregular, discontinuous, and acutely marginated, a ductus diverticulum is smoothly contoured with obtuse margins and will lack any surrounding hematoma [3, 15, 16] (Fig. 18). Occasionally, a fibrotic remnant of the ductus arteriosus can persist and may calcify along the undersurface of the aorta and should also not be confused with ATAI. The aortic spindle is another normal anatomic variant potentially simulating ATAI. A remnant of the appearance of the fetal aorta, the aortic spindle is a smooth dilatation of the proximal descending aorta just past the isthmus. It is fusiform rather than saccular in appearance, distinguishing it from a ductus diverticulum or aortic injury [17] (Fig. 19).
Oblique sagittal reformatted chest CT with intravenous contrast image demonstrates a smoothly contoured, obtusely marginated outpouching along the anterior aspect of the aortic isthmus (arrow), consistent with a ductus diverticulum, a normal anatomic variant which should not be mistaken for traumatic aortic injury
Diagnosis, Classifying, and Reporting ATAI
In cases of ATAI with direct imaging findings on CT, confirmation with conventional angiography is not required and will only serve to delay management. Furthermore, CT may better identify subtle injuries that could be difficult or impossible to detect on conventional angiography [12]. Using a combination of direct and indirect imaging findings can allow the radiologist to confidently diagnose ATAI on CT with a high sensitivity and specificity [1, 7, 18, 19].
For cases in which there is a mediastinal hematoma without direct findings of ATAI, there are several appropriate follow-up options. In stable patients, it is reasonable to recommend a repeat CT scan in 48–72 h to assess for evolution of potential ATAI. Alternatively, transcatheter intravascular ultrasound or transesophageal echocardiography can be recommended to evaluate for subtle CT-occult injury. Conventional angiography was previously utilized in these scenarios; however, in the era of high-resolution MDCT, angiography is unlikely to provide any additional useful information [2]. Furthermore, angiography is disadvantageous to CT given its invasive nature, risk of access site complications, and the need to transfer the patient to the angiography suite or operating room. It should be noted that angiograms are useful to the vascular surgeons in the operating room at the time of definitive endoluminal repair.
Several different classification systems have been proposed to grade the severity of aortic injury and secondary findings. The most widely used classification system that was adopted by the Society of Vascular Surgery divides aortic injury into 4 grades: grade I (intimal tears); grade II (intramural hematoma); grade III (pseudoaneurysm); and grade IV (aortic rupture) [20]. Other authors have adapted this scheme and further subdivided injuries based on the size of the intimal flap or pseudoaneurysm [21,22,23,24,25]. Although radiologists should be aware of these categories when reporting the findings of ATAI, accurate grading remains challenging, and attempts to do so may be potentially misleading by inadvertently underestimating the true extent of injury.
At our institution, we prefer to describe the injury by giving attention to the key information needed to guide treatment decisions, specifically the presence or absence of a mediastinal/periaortic hematoma, the size of any tissue flaps, luminal thrombus, focal outpouchings, intramural hematoma, and the presence or absence of active contrast extravasation. The concept of “minimal aortic injury” has been reported in the literature, with many studies characterizing it as a small (less than 1 cm) tissue flap, thrombus, or intramural hematoma, without associated mediastinal hematoma or focal outpouching suggesting a pseudoaneurysm [23, 24, 26,27,28,29,30,31,32,33]. Yet, there remains no uniform definition of “minimal aortic injury,” and, as such, we discourage the use of this terminology.
Management and Treatment of ATAI
Although classification systems of ATAI have their limitations, stratification by injury severity helps to guide management and predict prognosis [23]. Low-grade injuries may resolve completely with non-operative, medical management alone using a combination of heart rate and blood pressure control along with anticoagulation and antiplatelet therapy [25, 34, 35], an approach reflected in the Society of Vascular Surgery recommendation for expectant management of grade I injuries [20]. Recent studies have suggested that this conservative management style may extend to grade II and potentially grade III injuries in selected patients [22, 26,27,28,29,30,31,32], [36•]. At this point, however, we cannot recommend this approach given the paucity of long-term observational data regarding the stability of these unrepaired higher-grade lesions that, as some have shown, may progress to chronic vascular injury or frank aortic rupture months or years after the inciting traumatic event [37, 38]. With this in mind, the exact duration and interval for follow-up imaging of low-grade injuries managed conservatively or periaortic hematoma in the absence of direct signs of ATAI remains unresolved. Generally, most patients should receive a follow-up CT in 48–72 h following initial presentation and, if not completely resolved, will require long-term serial follow-up imaging to ensure stability or improvement over time (Fig. 20).
Oblique sagittal reformatted chest CT with intravenous contrast image (a) in this patient status post motor vehicle collision demonstrates a small thrombus arising from the anterior wall of the aorta at the level of the isthmus (ellipse). No periaortic hematoma was identified, and no additional signs of direct aortic injury were visualized. The patient underwent conservative management and repeat examination 48 h later (b) reveals resolution of the low-grade injury
High-grade ATAI is typically treated with endovascular repair, which is associated with lower rates of mortality, paraplegia, and procedural morbidity compared to open repair [25, 39], [40••], [41, 42]. However, the timing of repair remains debatable. While the Society of Vascular Surgery currently recommends urgent (less than 24 h) endovascular repair for grade II–IV injuries [20], recent studies have reported on the increased use of delayed repair, particularly in patients with moderate/severe traumatic brain injury [39, 41], [43•], [44]. This approach is reasonable, considering that ATAI is not always found in isolation and is often associated with numerous additional life-threatening injuries that may require more immediate intervention and stabilization [22]. Unsurprisingly, according to the latest Eastern Association for the Surgery of Trauma (EAST) workgroup guidelines, urgent repair is only indicated in cases of hemodynamic instability that can be specifically explained by ATAI. In cases of ATAI with hemodynamic stability, delayed repair is recommended until additional life-threatening injuries are appropriately managed [40••].
Several key measurements important for surgeons potentially planning endovascular repair should be detailed in radiology reports. These include the length of the injury, the aortic diameter proximal and distal to the injury, and, most importantly, the distance from the injury to the origin of the left subclavian artery, as the lack of an adequate proximal landing zone may require covering the left subclavian artery and a subsequent left carotid-subclavian artery bypass [5]. In addition, consideration should be given to any variant aortic arch anatomy, prior thoracic surgical changes, and reduced diameter of access vessels, as any of these findings may dramatically alter the approach to operative or endovascular repair [45].
Follow-up imaging for repaired ATAI will depend on the type of treatment selected and there are no specific uniform protocols in place. Patients who undergo open or endovascular repair will require serial imaging, either with CT or MR, the latter of which is useful in minimizing radiation dose. Long-term follow-up is essential in this cohort of predominantly younger, otherwise healthy patients to ensure endograft stability and integrity, particularly as the aorta remodels and lengthens with age [21, 46].
Conclusions
ATAI is a life-threatening complication of blunt and penetrating chest trauma with often vague clinical signs and symptoms. The radiologist plays an integral role in making a prompt, life-saving diagnosis of ATAI and directing further management/treatment. The chest radiograph remains the initial screening examination in the setting of trauma and can be utilized to evaluate for immediate life-threatening injuries and signs of mediastinal hematoma, prompting further evaluation with contrast-enhanced chest CT. Mediastinal hematoma is an indirect finding of aortic injury and should result in careful scrutiny of the aorta for direct findings of injury. Direct findings of ATAI include contour irregularities of the aorta, caliber changes of the aorta, intraluminal thrombus or flaps, discontinuities of the aortic wall, or in rare cases frank extravasation of contrast. Once ATAI is diagnosed, it is essential for the radiologist to describe its appearance and location to facilitate appropriate management and treatment.
References
Recently published papers of particular interest have been highlighted as: • Of importance •• Of major importance
Steenburg SD, Ravenel JG, Scho C. Acute traumatic aortic injury: imaging evaluation and management. Radiology. 2008;248(3):748–62.
Sammer M, Wang E, Blackmore CC, Burdick TR, Hollingworth W. Indeterminate CT angiography in blunt thoracic trauma: is CT angiography enough? AJR Am J Roentgenol. 2007;189(3):603–8. http://www.ncbi.nlm.nih.gov/pubmed/17715106.
Dosios TJ, Salemis N, Angouras D, Nonas, E. Blunt and penetrating trauma of the thoracic aorta and aortic arch branches: an autopsy study. J Trauma Acute Care Surg. 2000;49(4). http://journals.lww.com/jtrauma/Fulltext/2000/10000/Blunt_and_Penetrating_Trauma_of_the_Thoracic_Aorta.18.aspx.
Demetriades D, Velmahos GC, Scalea TM, Jurkovich GJ, Karmy-Jones R, Teixeira PG, et al. Diagnosis and treatment of blunt thoracic aortic injuries: changing perspectives. J Trauma. 2008;64(6):1415–9.
Raptis C, Hammer M, Raman K, Mellnick V, Bhalla S. Acute traumatic aortic injury: practical considerations for the diagnostic radiologist. J Thorac Imaging. 2015;30(3):202–13. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=yrovftq&NEWS=N&AN=00005382-201505000-00006.
Woodring JH, Dillon ML. Radiographic manifestations of mediastinal hemorrhage from blunt chest trauma. In: Annals of Thoracic Surgery; 1984. Vol. 37, p. 171–8.
Mirvis SE, Shanmuganathan K, Miller BH, White CS, Turney SZ. Traumatic aortic injury: diagnosis with contrast-enhanced thoracic CT–5-year experience at a major trauma center. Radiology. 1996;200(2):413–22. http://www.ncbi.nlm.nih.gov/pubmed/8685335.
Zaw AA, Stewart D, Murry JS, Hoang DM, Sun B, Ashrafian S, et al. CT Chest with IV contrast compared with CT angiography after blunt trauma. In: American Surgeon; 2016. p. 41–5.
Mirvis SE, Shanmuganathan K, Buell J, Rodriguez A. Use of spiral computed tomography for the assessment of blunt trauma patients with potential aortic injury. J Trauma. 1998;45(5):922–30. http://www.ncbi.nlm.nih.gov/pubmed/9820704.
Gavant ML, Flick P, Menke P, Gold RE. CT aortography of thoracic aortic rupture. Am J Roentgenol. 1996;166(4):955–61.
Scaglione M, Pinto A, Pinto F, Romano L, Ragozzino A, Grassi R. Role of contrast-enhanced helical CT in the evaluation of acute thoracic aortic injuries after blunt chest trauma. Eur Radiol. 2001;11(12):2444–8.
Mirvis SE, Shanmuganathan K. Diagnosis of blunt traumatic aortic injury 2007: still a nemesis. Eur J Radiol. 2007;2007:27–40.
Cleverley JR, Barrie JR, Raymond GS, Primack SL, Mayo JR. Direct findings of aortic injury on contrast-enhanced CT in surgically proven traumatic aortic injury: a multi-centre review. Clin Radiol. 2002;57(4):281–6.
Tsai R, Raptis D, Raptis C, Mellnick VM. Traumatic abdominal aortic injury—clinical considerations for the diagnostic radiologist. Abdom Radiol. 2018. https://doi.org/10.1007/s00261-018-1523-2.
Burkhart HM, Gomez GA, Jacobson LE, Pless JE, Broadie TA. Fatal blunt aortic injuries: a review of 242 autopsy cases. J Trauma Acute Care Surg. 2001;50(1):113–5.
Cook CC, Gleason TG. Great vessel and cardiac trauma. In: Surgical Clinics of North America; 2009. Vol. 89, p. 797–820.
Fisher RG, Sanchez-Torres M, Whigham CJ, Thomas JW. “Lumps” and “bumps”; that mimic acute aortic and brachiocephalic vessel injury. Radiographics. 1997;17(4):825–34. http://www.ncbi.nlm.nih.gov/pubmed/9225385.
Fabian TC, Davis KA, Gavant ML, Croce MA, Melton SM, Patton JH, et al. Prospective study of blunt aortic injury: helical CT is diagnostic and antihypertensive therapy reduces rupture. In: Annals of Surgery; 1998. p. 666–77.
Gavant ML, Menke PG, Fabian T, Flick PA, Graney MJ, Gold RE. Blunt traumatic aortic rupture: detection with helical CT of the chest. Radiology. 1995;197(1):125–33.
Lee WA, Matsumura JS, Mitchell RS, Farber MA, Greenberg RK, Azizzadeh A, et al. Endovascular repair of traumatic thoracic aortic injury: clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg. 2011;53(1):187–92.
Alberta HB, Secor JL, Smits TC, Farber MA, Jordan WD, Azizzadeh A, et al. Comparison of thoracic aortic diameter changes after endograft placement in patients with traumatic and aneurysmal disease. J Vasc Surg. 2014;59(5):1241–6.
Rabin J, DuBose J, Sliker CW, O’Connor J V., Scalea TM, Griffith BP. Parameters for successful nonoperative management of traumatic aortic injury. J Thorac Cardiovasc Surg. 2014;147(1):143–50. http://linkinghub.elsevier.com/retrieve/pii/S0022522313009732.
Lamarche Y, Berger FH, Nicolaou S, Bilawich AM, Louis L, Inacio JR, et al. Vancouver simplified grading system with computed tomographic angiography for blunt aortic injury. J Thorac Cardiovasc Surg. 2012;144(2):347–54.
Gavant ML. Helical CT grading of traumatic aortic injuries: Impact on clinical guidelines for medical and surgical management. Radiologic Clinics of North America. 1999. Vol. 37, p. 553–74.
Heneghan RE, Aarabi S, Quiroga E, Gunn ML, Singh N, Starnes BW. Call for a new classification system and treatment strategy in blunt aortic injury. J Vasc Surg. 2016;64(1):171–6.
Starnes BW, Lundgren RS, Gunn M, Quade S, Hatsukami TS, Tran NT, et al. A new classification scheme for treating blunt aortic injury. J Vasc Surg. 2012;55(1):47–54.
Paul JS, Neideen T, Tutton S, Milia D, Tolat P, Foley D, et al. Minimal aortic injury after blunt trauma: selective nonoperative management is safe. In: Journal of Trauma—Injury, Infection and Critical Care; 2011. p. 1519–23.
Mosquera VX, Marini M, Gulías D, Cao I, Muñiz J, Herrera-Noreña JM, et al. Minimal traumatic aortic injuries: meaning and natural history. In: Interactive Cardiovascular and Thoracic Surgery. 2012. Vol. 14, p. 773–8.
Malhotra AK, Fabian TC, Croce MA, Weiman DS, Gavant ML, Pate JW. Minimal aortic injury: a lesion associated with advancing diagnostic techniques. J Trauma. 2001;51(6):1042–8. http://www.ncbi.nlm.nih.gov/pubmed/11740248.
Kepros J, Angood P, Jaffe CC, Rabinovici R. Aortic intimal injuries from blunt trauma: resolution profile in nonoperative management. In: Journal of Trauma. 2002. Vol. 52, p. 475–8.
Forman MJ, Mirvis SE, Hollander DS. Blunt thoracic aortic injuries: cT characterisation and treatment outcomes of minor injury. Eur Radiol. 2013;23(11):2988–95.
Caffarelli AD, Mallidi HR, Maggio PM, Spain DA, Miller DC, Mitchell RS. Early outcomes of deliberate nonoperative management for blunt thoracic aortic injury in trauma. J Thorac Cardiovasc Surg. 2010;140(3):598–605. https://doi.org/10.1016/j.jtcvs.2010.02.056.
Azizzadeh A, Keyhani K, Miller CC, Coogan SM, Safi HJ, Estrera AL. Blunt traumatic aortic injury: initial experience with endovascular repair. J Vasc Surg Off Publ Soc Vasc Surg [and] Int Soc Cardiovasc Surgery, North Am Chapter. 2009;49(6):1403–8. http://www.ncbi.nlm.nih.gov/pubmed/21129901.
Osgood MJ, Heck JM, Rellinger EJ, Doran SL, Garrard CL, Guzman RJ, et al. Natural history of grade I–II blunt traumatic aortic injury. In: Journal of Vascular Surgery. 2014. p. 334–42.
Gunn MLD, Lehnert BE, Lungren RS, Narparla CB, Mitsumori L, Gross JA, et al. Minimal aortic injury of the thoracic aorta: imaging appearances and outcome. Emerg Radiol. 2014;21(3):227–33.
•Gandhi SS, Blas JV., Lee S, Eidt JF, Carsten CG. Nonoperative management of grade III blunt thoracic aortic injuries. J Vasc Surg. 2016;64(6):1580–6. Exploring the benefits of delayed management for a grade III injury, which are typically repaired.
Mosquera VX, Marini M, Lopez-Perez JM, Muñiz-Garcia J, Herrera JM, Cao I, et al. Role of conservative management in traumatic aortic injury: comparison of long-term results of conservative, surgical, and endovascular treatment. J Thorac Cardiovasc Surg. 2011;142(3):614–21. http://linkinghub.elsevier.com/retrieve/pii/S0022522310012766.
Finkelmeier BA, Mentzer RMJ, Kaiser DL, Tegtmeyer CJ, Nolan SP. Chronic traumatic thoracic aneurysm. Influence of operative treatment on natural history: an analysis of reported cases, 1950–1980. J Thorac Cardiovasc Surg. 1982;84(2):257–66.
Demetriades D, Velmahos GC, Scalea TM, Jurkovich GJ, Karmy-Jones R, Teixeira PG, et al. Blunt traumatic thoracic aortic injuries: early or delayed repair—results of an american association for the surgery of trauma prospective study. J Trauma Inj Infect Crit Care. 2009;66(4):967–73. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00005373-200904000-00001.
••Fox N, Schwartz D, Salazar JH, Haut ER, Dahm P, Black JH, et al. Evaluation and management of blunt traumatic aortic injury: A practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2015;78(1):136–46. Comphrensive guidelines for ATAI surgical management, which recommends delayed repair in cases of ATAI with hemodynamic stability.
De Mestral C, Dueck A, Sharma SS, Haas B, Gomez D, Hsiao M, et al. Evolution of the incidence, management, and mortality of blunt thoracic aortic injury: a population-based analysis. J Am Coll Surg. 2013;216(6):1110–5.
DuBose JJ, Leake SS, Brenner M, Pasley J, O’Callaghan T, Luo-Owen X, et al. Contemporary management and outcomes of blunt thoracic aortic injury: a multicenter retrospective study. J Trauma Acute Care Surg. 2015;78(2):360–9.
•Rabin J, Harris DG, Crews GA, Ho M, Taylor BS, Sarkar R, et al. Early aortic repair worsens concurrent traumatic brain injury. In: Annals of Thoracic Surgery. 2014. p. 46–52. Study supporting the use of delayed repair in patients with ATAI with life threatening co-morbidities, specifically traumatic brain injury.
Di Eusanio M, Folesani G, Berretta P, Petridis FD, Pantaleo A, Russo V, et al. Delayed management of blunt traumatic aortic injury: open surgical versus endovascular repair. Ann Thorac Surg. 2013;95(5):1591–7. https://doi.org/10.1016/j.athoracsur.2013.02.033.
Nagpal P, Mullan BF, Sen I, Saboo SS, Khandelwal A. Advances in imaging and management trends of traumatic aortic injuries. Cardiovasc Intervent Radiol. 2017;40(5):643–54.
Forbes TL, Harris JR, Lawlor DK, DeRose G. Aortic dilatation after endovascular repair of blunt traumatic thoracic aortic injuries. J Vasc Surg. 2010;52(1):45–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. Zak Rajput, Demetrios A. Raptis, Constantine A. Raptis, and Sanjeev Bhalla each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical collection on Emergency Radiology.
Rights and permissions
About this article
Cite this article
Rajput, M.Z., Raptis, D.A., Raptis, C.A. et al. Imaging of Acute Traumatic Aortic Injury. Curr Radiol Rep 6, 19 (2018). https://doi.org/10.1007/s40134-018-0278-4
Published:
DOI: https://doi.org/10.1007/s40134-018-0278-4