Abstract
Colorectal cancer is a common malignancy that most commonly metastasizes to the liver. There has been considerable effort in developing new treatment options for these patients. One method that has been developed for the treatment of colorectal metastases to the liver is irinotecan-loaded drug-eluting bead (DEBIRI) embolization. This article reviews the current literature on DEBIRI and discusses the state of current knowledge and possible areas of future investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The liver is the site of spread in 70% of patients with metastatic colorectal cancer [1]; in 50% of these patients, metastatic disease will be the primary cause of death [2]. The accepted first line therapy for liver-only metastatic disease is surgical resection; however, only 20–30% [3, 4] of patients will be resection candidates, and of those who undergo resection 70–80% will have a recurrence of metastatic disease within 5 years [5].
In patients with unresectable disease, the first line therapy has been chemotherapy. The development of FOLFOX (leucovorin, 5-Fluorouracil, and oxaliplatin) and FOLFIRI (leucovorin, 5-Fluorouracil, and irinotecan) resulted in significant improvements in survival [6]. The available chemotherapeutic agents have continued to grow as has knowledge of the disease, with targeted therapy being actively developed. Survival times have been further improved with the addition of biologics, with both anti-epidermal growth factor receptor (EGFR), such as Cetuximab and Panitumumab, and anti-vascular endothelial growth factor (VEGF) antibodies, such as Bevacizumab, Aflibercept, Ramucirumab, and Regorafenib showing some efficacy [6, 7]. The ability to tailor treatment regiments has improved as well; for instance, recent studies have indicated KRAS and NRAS mutations are negative predictors for anti-EGFR therapy [7]. However, even with these advances, most studies have found a median overall survival (OS) rate in the range of 18–29 months [8] as measured from the initiation of chemotherapy. While considerable advances have been, and continue to be made, in the treatment of colorectal metastatic disease, further improvement is needed.
One area of active study is the use of irinotecan drug-eluting beads (DEBIRI). Herein, we review the available literature on the efficacy and safety of DEBIRI. We also review pertinent technical articles that aim to optimize the DEBIRI technique.
DEBIRI Mechanism of Action and Technique Considerations
DEBIRI is commonly performed in patients with metastatic colorectal cancer, who are not considered to be surgical or ablation candidates. Patients are typically considered to be DEBIRI candidates when they have liver-only or liver-dominant metastatic disease. The use of DEBIRI as first line, second line, or salvage therapy has varied widely in the literature with no clear consensus regarding its appropriate place. Studies have investigated the use of DEBIRI concomitantly with multiple chemotherapeutic regiments including FOLFOX [9, 10], cetuximab [11], and capecitabine [12], and those authors concluded these approaches were safe.
Irinotecan is a topoisomerase inhibitor and a semisynthetic analog of camptothecin, which requires activation by normal liver cells. Activation is accomplished by the enzymes carboxylesterase 1 (CES-1) and carboxylesterase 2 (CES-2) that convert the drug into its active form 7-Ethyl-10-hydroxy-camptothecin (SN-38) [13]. SN-38 works by inhibiting topoisomerase I which in turn inhibits DNA replication and transcription.
Irinotecan can be loaded onto microspheres and then delivered to tumors via the hepatic arteries. However, in contrast to transarterial chemoembolization (TACE) utilized in the treatment of other cancers, sub-selective embolization is typically not recommended for two reasons. The first is the fact that irinotecan requires activation by normal liver parenchyma via hydrolysis. The second reason for lobar delivery of the drug is the desire to treat all metastatic disease, including radiographic occult lesions.
While previous authors have utilized various dosing strategies, a recent panel of experts recommended two treatment sessions 3–4 weeks apart with delivery of 100 mg of irinotecan at each session [14]. However, it should be noted that no good dose escalation studies have been performed to determine the ideal dose of irinotecan. If both lobes are affected, it is recommended that each lobe be treated twice for a total of four treatments where alternate lobes are treated every 2 weeks. Finally, based on a retrospective review of a prospectively maintained registry [15] that demonstrated decreased adverse events (AEs) and increased dose delivery when using small (75–150 or 100–300 µm) as compared to large (100–300 or 500–700 µm) beads, most advocate the use of smaller beads.
Published Data
Table 1 briefly reviews all of the published clinical trials.
Randomized Controlled Trials
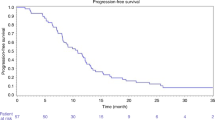
Martin et al. [9, 10] published a phase I followed by a phase II study evaluating the safety and tumor response rate of DEBIRI in combination with systemic modified FOLFOX ± bevacizumab as compared to FOLFOX ± bevacizumab alone in chemotherapy naïve patients. Seventy patients (40 in the DEBIRI arm and 30 in the control arm) were enrolled, with the first 10 undergoing DEBIRI and FOLFOX ± bevacizumab to assess safety. Patients with liver-dominant disease (>80% of total metastatic disease) were treated with the aim of delivering 100 mg of irinotecan-loaded 100–300 µm LC Beads® (BTG, London, UK) per session. The patients received at least 12 cycles of FOLFOX ± bevacizumab and two cycles of DEBIRI during chemotherapy off weeks. The technical success rate (defined as ability to deliver at least 75 mg of DEBIRI) was 84% and they delivered DEBIRI in a lobar fashion. A significantly increased number of serious adverse events (SAEs) (p = 0.03) in the DEBIRI arm compared to the control cohort was found. However, a significant improvement in overall response rates (ORR) at 6 months (p = 0.05) and liver progression-free survival (p = 0.05) was seen in the DEBIRI arm. The study failed to show significant improvement in progression-free survival (PFS) (p > 0.05) and median overall survival (OS) had not been reached at the time of publication (24 months).
In 2012, Fiorentini et al. [16] reported their findings in a prospective randomized controlled trial comparing DEBIRI with systemic irinotecan in patients who had not previously received irinotecan. Seventy-four patients with liver-only metastatic disease were randomized (36 to DEBIRI and 38 to FOLFIRI arms), all of whom had received 2–3 lines of prior chemotherapy. The study aimed to deliver 200 mg of irinotecan with 100–300 µm LC beads® two times with a month interval for the DEBIRI cohort. The FOLFIRI arm received a total of 8 cycles. Increased OS in the DEBIRI group compared to the FOLFIRI group (OS median 22 and 15 months respectively, p = 0.031) was found. PFS was also longer in the DEBIRI group (median 7 months) as compared to the FOLFIRI group (4 months) (p = 0.006). This study demonstrated different toxicity profiles in the two cohorts with significantly less occurrences of neutropenia and mucositis in the DEBIRI as compared to FOLFIRI cohorts (p < 0.0001 and p = 0.00002 respectively). Lastly, a significantly better quality of life in the DEBIRI compared to FOLFIRI groups was found at 1 (p = 0.038), 3 (p = 0.025), and 8 (p = 0.025) months. However, this group did not include cetuximab in the FOLFIRI arm which has drawn some criticism. In order to address this concern, Fiorentini et al. [11] subsequently published a prospective trial of 40 patients in which participants were concomitantly treated with DEBIRI and cetuximab. AEs were seen in 10 patients with 4 of these patients experiencing SAEs. All AEs were related to skin issues, and included acne-like rash, fissuring, dryness, and hypersensitivity. The authors did not consider post-embolization syndrome symptoms to be AEs; however, symptoms consistent with post-embolization syndrome were seen in 12 (30%) patients. Encouraging PFS (9.8 months) and OS (20.4 months) endpoints were demonstrated.
Prospective Single-arm Studies
Authors of a multicenter registry investigating the use of DEBIRI in patients who had received between 0 and 4 prior lines of chemotherapy have published their data multiple times [12, 17]. Patients with liver-dominant disease, defined as >50% of metastatic burden, were treated, with a goal of delivering 200 mg of irinotecan to each affected lobe. At least 50% of the desired dose was delivered to each lobe in all 666 treatments performed in 296 patients. Analysis was performed by dividing the group of 296 patients into several sub groups. There was no significant difference in ORR, AEs, and OS in patients who had and had not received systemic irinotecan. They also assessed differences in patients based on the number of prior lines of chemotherapy they had received and found minimal differences in AEs (between no prior lines of chemotherapy and one (p = 0.0039) and three lines (p = 0.0062)). Minor differences in response rates at various time points, the most notable of which was significant improvement in complete response (CR) at 12 months in chemotherapy naïve patients as compared to those who had 1(p = 0.0069), 2 (p = 0.013), and 3 (p = 0.054) lines of chemotherapy, were also demonstrated.
In 2012 a German group published their findings in 11 patients recruited for a single-arm prospective safety study [21]. This group treated patients with liver-only disease who had been off chemotherapy for at least 4 weeks. Patients were treated every 3 weeks for a total of 4 treatments in a supra-selective manner. Treatments aimed to deliver a dose of up to 400 mg of irinotecan loaded onto 100–300 µm or 300–500 µm DC Beads™ (BTG, London, UK). Of the 11 patients, 9 received all 4 treatments with two patients withdrawing after 2 treatments (1 for intrahepatic progression and the other due a vascular abnormality leading to shunting to the heart). This group experienced no SAEs, but did have 41 AEs in 9/11 patients. The majority of AEs (63%) were signs and symptoms consistent with post-embolization syndrome (pain, etc.). Response was measured by Response Evaluation Criteria in Solid Tumors (RECIST). By the end of the study period, 7/11 (83%) showed progressive disease, 2/11 (18%) showed partial response, and 2/11 (18%) showed stable disease.
Iezzi et al. [22] reported their results of a single-arm prospective Phase II arm study that aimed to evaluate the safety and efficacy of DEBIRI plus capecitabine in patients who had disease progression despite two or more lines of chemotherapy. Their cohort of 20 patients, included those with liver-dominant disease, defined as >80% of metastatic burden confined to the liver. M1 (< 150 µm) DC Beads™ loaded with 100 mg of irinotecan were used. The treatment schedule differed for unilobar versus bilobar disease, with a single lobe treated at a time. For unilobar disease, they treated one lobe in two sessions with a 4 weeks interval between treatments. For bilobar disease, they treated one lobe every 2 weeks for a total of 4 treatments with 4 weeks between treatments of the same lobe. On the third day following the first DEBIRI treatment, patients started taking 1000 mg/m2 of capecitabine orally twice a day for 2 weeks with 1 week off until unacceptable side effects or disease progression occurred. The planned DEBIRI protocol was not completed in 8/40 (20%) of patients secondary to clinical progression (5/8), withdrawal of consent (2/8), and grade 3 AE of vasospastic angina (1/8). Sixty-six AEs were experienced that were all mild (grade 1–2), except for 2 grade 3 AEs (nausea/vomiting and acute vasospastic angina). The cohorts PFS and OS were 4 and 7.3 months, respectively.
Aliberti et al. [23] published their single-arm prospective phase II trial of 82 patients whom had failed at least two prior lines of chemotherapy. The number of patients who had extrahepatic disease was not reported. Patients were treated in a lobar or segmental fashion with 100–200 mg of irinotecan loaded onto 100–300 or 300–500 µm DC Beads™. Delivery of 100% of the intended dose was achieved in all patients. The majority of AEs in this study were mild (grade 1–2) events consistent with post-embolization syndrome. However, 20/82 (24%) patients experienced grade 3 right upper quadrant pain. An OS of 25 months and time to progression (TTP) of 8 months was reported. The study demonstrated a general improvement in quality of life (QoL) in 75/82 (90%) of patients that lasted 32 weeks. This group also reported two patients who were able to undergo successful hepatic resection after treatment.
Retrospective Studies
Narayanan et al. [24] published a retrospective study of 28 patients, 93% of whom had undergone at least one prior line of chemotherapy. They reported 75 total AEs in 47 procedures performed; the vast majority was mild (grade 1–2) with only 4 cases of grade 3 toxicity (2 cases of abdominal pain and 2 cases of hypertension). The mean and median PFS were 5.2 and 4.0 months, respectively, while the mean and median OS were 14.1 and 13.3 months, respectively.
Current State and Moving Forward
The initial DEBIRI studies have been promising. However, the current data are heterogeneous and have varied not only in basic technique but patient selection as well. The lack of standardization was noted as in a recent consensus statement on the treatment of colorectal metastases [25]. It will be important to further investigate the optimal timing for patients to receive DEBIRI during treatment for metastatic colorectal cancer. The strongest evidence thus far is the prospective randomized controlled trial by Fiorentini et al. [16] that demonstrated significant improvement in PFS and OS for patients receiving DEBIRI as a salvage therapy as compared to those who underwent FOLFIRI. Multiple other studies have also demonstrated encouraging responses in a salvage setting (greater than two lines of chemotherapy); however, these studies are limited by their single-arm design with the lack of comparison to a salvage chemotherapy or yttrium 90 (y90) therapy reducing these studies’ impact. The single study that investigated using DEBIRI as a first line therapy in concert with FOLFOX, a prospective randomized controlled trial, failed to show improvement in PFS and did not present data on OS. This would suggest that DEBIRI may be better reserved for second line therapy or later; however, this area would benefit from further investigation.
Another area of variance in the studies performed so far is whether to treat patients with liver-only or liver-dominant metastatic disease. The definition of liver-dominant disease has also varied significantly from >50 to >80% of total metastatic burden. Well-designed prospective studies to evaluate the benefit of DEBIRI in patients who have extrahepatic disease are needed to determine if any OS and PFS benefits exist.
The basic technique of DEBIRI is also yet to be well studied. While a recent panel of experts recommended an attempted dose delivery of 100 mg [14] per session, no dose escalation or comparative studies have been performed. Without further studies, the ideal target dose remains unknown. Optimal bead size also lacks clarity. There are limited data to suggest that smaller beads (75–150 or 100–300 µm) provide increased efficacy compared to larger beads (100–300 or 500–700 µm); however, the Akinwande et al. [15] study on which this is based, includes 100–300 µm LC Beads® in both the large and small bead cohorts. The improved outcomes seen in the Akinwande study may have been secondary to their ability to deliver a higher dose of irinotecan with the smaller particles. Interestingly, despite delivering a higher dose of irinotecan, the smaller bead cohort experienced fewer adverse events. While this evidence leads a panel of experts to recommend small bead sizes [14], a prospective randomized controlled trial is needed to thoroughly answer the question. Lastly, while it is believed that lobar distribution is best, given the desire to treat radiographically occult disease and the need for normal hepatocytes to activate the drug, this has not been conclusively proven.
Many patients have been reported to have significant pain associated with the procedure. This has made some providers hesitant to adapt DEBIRI. Various technical modifications have been proposed to improve patients’ pain, including intra-arterial lidocaine administration. However, this pain and other AEs may in part be related to the rate of irinotecan release by the particle used to deliver it. Further investigation into release times and possible modifications would be of benefit.
Selective internal radiation therapy has been developed over the last 15 years and is frequently used to treat this same patient population. Comparison of these two techniques to determine the comparative effectiveness and adverse event profile is needed. These studies will require careful design given the ever-changing landscape of chemotherapies available to these patients.
Conclusion
DEBIRI is a promising locoregional therapy option with great potential. The available data support its use in patients with liver metastases from colorectal cancer after failure of systemic chemotherapy. Given the promising initial data further randomized controlled trials to determine the ideal patient population, the most efficacious timing of treatment, as well as the optimal technique for bead delivery, is warranted.
References
Welch JP, Donaldson GA. The clinical correlation of autopsy study of recurrent colorectal cancer. Ann Surg. 1979;189:496–502.
Silverberg E. Cancer statistics, 1977. CA Cancer J Clin. 1977;27:26–41.
Stangl R, Altendorf-Hofmann A, Charnley RM, Scheele J. Factors influencing the natural history of colorectal liver metastases. Lancet. 1994;343:1405–10.
Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg. 1995;19:59–71.
Fong Y. Hepatic colorectal metastasis: current surgical therapy, selection criteria for hepatectomy, and role for adjuvant therapy. Adv Surg. 2000;34:351–81.
Sinicrope A, Okamoto K, Kasi PM, Kawakami H. Molecular biomarkers in the personalized treatment of colorectal cancer. Clin Gastroenterol Hepatol. 2016;14(5):651–8.
Ohhara Y, Fukuda N, Takeuchi S, et al. Role of targeted therapy in metastatic colorectal cancer. World J Gastrointest Oncol. 2016;8(9):642–55.
Gustavsson B, Carlsson G, Machover D, et al. A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clin Colorectal Cancer. 2015;14(1):1–10.
Martin RC 2nd, Scoggins CR, Tomalty D, et al. Irinotecan drug-eluting beads in the treatment of chemo-naive unrespectable colorectal liver metastasis with concomitant systemic fluorouracil and oxaliplatin: results of pharmacokinectics and phase I trial. J Gastrointest Surg. 2012;16(8):1531–8.
Martinc RC 2nd, Scoggins CR, Schreeder M, et al. Randomized controlled trial of irinotecan drug-eluting beads with simultaneous FOLFOX and bevacizumab for patients with unresectable colorectal liver-limited metastasis. Cancer. 2015;121(20):3649–58.
Fiorentini G, Aliberti C, Sarti D, et al. Locoregional therapy and systemic cetuximab to treat colorectal liver metastases. World J Gastrintest Oncol. 2015;7(6):47–54.
Akiwande O, Miller A, Hayes D, O’Hara R, Tomalty D, Martin RC. Concomitant capecitabine with hepatic delivery of drug eluting beads in metastatic colorectal cancer. Anticancer Res. 2014;34(12):723–7245.
Liu DM, Thakor AS, Baerlocher M, et al. A review of conventional and drug-eluting chemoembolization in the treatment of colorectal liver metastases: principles and proof. Future Oncol. 2015;11(9):1421–28.
Lencioni R, Aliberti C, de Baere T, et al. Transartrial treatment of colorectal cancer liver metastases with irinotecan-loaded drug-eluting beads: technical recommendations. J Vasc Interv Radiol. 2014;25(3):365–9.
Akinwande OK, Philips P, Duras P, Pluntke S, Scoggins C, Marin RC. Small versus large-sized drug-eluting beads (DEBIRI) for the treatment of hepatic colorectal metastases: a propensity score matching analysis. Cardiovasc Interv Radiol. 2015;38(2):361–71.
Fiorentini G, Aliberti C, Tilli M, et al. Intra-arterial infusion of irinotecan-loaded drug-eluting beads (DEBIRI) versus intravenous therapy (FOLFIRI) for hepatic metastases from colorectal cancer: final results of a phase III study. Anticancer Res. 2012;32(4):1387–95.
Martin RC, Joshi J, Robbins K, Tomalty D, O’Hara R, Tatum C. Transarterial chemoembolization of metastatic colorectal carcinoma with drug-eluting beads, irinotecan (DEBIRI): multi-institutional registry. J Oncol. 2009;2009:539795.
Martin RC, Joshi J, Robbins K, et al. Hepatic intra-arterial injection of drug-eluting bead, irinotecan (DEBIRI) in unresectable colorectal liver metastases refractory to systemic chemotherapy: results of multi-institutional study. Ann Surg Oncol. 2011;18(1):192–8.
Bhutiani N, Akinwande O, Martin RC 2nd, Efficacy and toxicity of hepatic intra-arterial drug-eluting (irinotecan) bead (DEBIRI) therapy in irinotecan-refractory unresectable colorectal liver metastases. World J Surg. 2016;40(5):1178–90.
Akinwande O, Scoggins C, Martin RC. Early experience with 70–150 µm irinotecan drug-eluting beads (M1-DEBIRI) for the treatment of unresectable hepatic colorectal metastases. Anticancer Res. 2016;36(7):3413–8.
Eichler K, Zangos S, Mack MG, et al. First human study in treatment of unresectable liver metastases from colorectal cancer with irinotecan-loaded beads (DEBIRI). J Oncol. 2012;41(4):1213–20.
Iezzi R, Marisico VA, Guerra A, et al. Trans-arterial chemoembolization with irinotecan-loaded drug-eluting beads (DEBIRI) and capecitabine in refractory liver prevalent colorectal metastases: a phase II single-center study. Cardiovasc Inerv Radiol. 2015;38(6):1523–31.
Aliberti C, Fiorentini G, Muzzio PC, et al. Trans-arterial chemoembolization of metastatic colorectal carcinoma to the liver adopting DC Bead®, drug-luting bead loaded with irinotecan: results of a phase II clinical study. Anticancer Res. 2011;31(12):4581–7.
Narayanan G, Barbery K, Suthar R, Guerrero G, Arora G. Transarterial chemoembolization using DEBIRI for treatment of hepatic metastases from colorectal cancer. Anticancer Res. 2013;33(5):2077–83.
Abdalla EK, Bauer TW, Chun YS, D’Angelica M, Kooby DA, Jarnagin WR. Locoregional surgical and interventional therapies for advanced colorectal cancer liver metastases: expert consensus statements. HPB(Oxford). 2013;15(2):119–30.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Neither Dr. Young, Dr. D’Souza, Dr. Flanagan, nor Dr. Golzarian have any pertinent conflicts of interest to report.
Rights and permissions
About this article
Cite this article
Young, S., D’Souza, D., Flanagan, S. et al. Review of the Clinical Evidence for the Use of DEBIRI in the Treatment of Colorectal Metastatic Disease. Cardiovasc Intervent Radiol 40, 496–501 (2017). https://doi.org/10.1007/s00270-016-1537-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-016-1537-5