Abstract
Purpose
The primary objective of this study is to evaluate the safety, tolerance, and pharmacokinetic profile of liver-directed therapy with drug-eluting beads irinotecan (DEBIRI) in combination with systemic modified FOLFOX in the treatment of unresectable liver metastases in chemotherapy-naive patients with colorectal cancer.
Design
DEBIRI, loaded with 100 mg irinotecan (100–300 μm beads), was administered via hepatic artery during the off week of FOLFOX therapy. Primary endpoints were safety, tolerance, systemic dose-limiting toxicities, and pharmacokinetics of systemic irinotecan and its active metabolite SN-38 at each infusion at 1-, 4-, and 24-h post-DEBIRI. Secondary endpoints were response rate and survival.
Results
The ten patients have undergone at least 12 cycles of FOLFOX in combination with at least two DEBIRI bead treatments during the patients’ off week. Pharmacokinetic data has demonstrated minimal detectable levels of irinotecan (18.6, 21, and 18.6 ng/ml) and SN-38 (1.06, 1.47, and 1.55 ng/ml) after the first, second, and third DEBIRI treatments, respectively. Currently, there has been only one severe device-related adverse event, a grade 3 hypertensive episode that required 1 day of observation in the hospital. The initial 9- and 12-month response rates have been 100 % (2 CR, 8 PR). Four (40 %) patients were successfully downstaged to resection and/or ablation with a median overall survival of 15.2 months.
Conclusion
Concomitant DEBIRI and FOLFOX±bevacizumab is safe, with a minimal adverse event rate, no dose-limiting toxicities, and enhanced overall response rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For colorectal cancer, up to 70 % of metastases occur in the liver1 and liver metastases represent a predominant cause (50 %) of death in these patients.2 At the time of detection of the primary tumor, 15–25 % of patients will have hepatic involvement and another 20 % will develop liver metastases following treatment of their primary cancer.3 Without any treatment, the median survival after detection of liver metastases is approximately 9 months, depending on the extent of the disease at the time of diagnosis.3
In patients with metastases confined to the liver, surgical resection, or complete tumor ablation remain the only treatments able to offer the prospect of long-term survival. After resection, a 5-year survival rate of 35–45 % can be achieved.4 However, only a small proportion of patients with liver metastases from colorectal cancer (MCRC) are suitable for surgery at the time of initial diagnosis. In the majority, liver metastases prove unresectable due to excessive tumor burden and/or unfavorable location of the hepatic lesions.5 In these patients, chemotherapy can be effective and has been shown to prolong median survival. More recently, with the introduction of irinotecan and oxaliplatin, chemotherapy regimens have achieved median survival times of up to 19 months6 and the addition of targeted therapies, such as bevacizumab and cetuximab, has further enhanced that to 24 months.
Oxaliplatin in combination with 5-fluorouracil and leucovorin (5-FU/LV) has emerged as an improved chemotherapy regimen for the treatment of advanced or metastatic colorectal carcinoma.7 In recent years, the dosing regimen of folinic acid, fluorouracil, and oxaliplatin (FOLFOX) has been modified in an attempt to make the treatment more convenient and to improve safety and efficacy. FOLFOX6 and modified FOLFOX6 (mFOLFOX6) have possibly provided marginal improvement in safety and efficacy over FOLFOX4 from the clinical evidence, to date, but there are no direct comparisons.8 Across a number of studies, FOLFOX4 and FOLFOX6 have consistently yielded response rates [response evaluation criteria in solid tumors (RECIST)] of 50–60 %, progression-free survival of 8–9 months, and median survival of 16–19 months.7 The difference between FOLFOX4 and FOLFOX6 is the continuous pump 5-FU is for 46 h for FOLFOX6 and 22 h for FOLFOX4. The efficacy of irinotecan in combination with infusional 5FU/LV (FOLFIRI) is comparable to that of FOLFOX in the setting of metastatic disease.9 However, continued concerns remain in regard to hepatic damage, steatohepatitis with oxaliplatin and sinusoidal occlusion with irinotecan, that we have demonstrated to be reduced with hepatic arterial therapy.14 More recently, there has been interest in combining all the above active agents into a FOLFOXIRI regimen, as was first reported by Falcone et al.,10 in a phase III study comparing FOLFIRI to FOLFOXIRI in 244 patients with unresectable MCRC. At a median follow-up of 18.4 months, they reported significant improvements in response rate, R0 resection of metastases, progression-free survival, and overall survival for the FOLFOXIRI arm. However, there was significantly greater neurotoxicity, neutropenia, and diarrhea in the treatment arm (83 % incidence), as well as patients receiving optimal dosing is only 65 % of patients.
From this report, it is obvious that a four-drug regimen is active, but with significant toxicity. Thus, the potential remains that if one of these active agents—irinotecan—could be delivered locally, the response benefits could be maintained without the added systemic toxicity. We have recently published on both the safety11 and efficacy12 of drug-eluting beads irinotecan (DEBIRI) as a single modality in the treatment of patients with unresectable metastatic colorectal cancer who had failed prior systemic therapy. We demonstrated enhanced response rates,13 without added systemic effects and the ability to downstage patients to hepatic resection.14,15
Thus, the primary objective for this study was to evaluate the safety and maximum tolerated dose of DEBIRI during concurrent modified FOLFOX6 (FOLFOXDEBIRI) in the first line setting in colorectal cancer and to establish the pharmacokinetics and systemic exposure of irinotecan and its active metabolite SN-38 in this setting.
Methods
A Federal Drug Administration investigation drug-exempted, University of Louisville IRB-approved prospective multi-institutional Pilot clinical evaluation of DEBIRI (LC/DC Bead®, Biocompatibles, UK) in combination with systemic mFOLFOX6 was performed from May 2009 to May 2010. Informed consent was obtained from all subjects prior to any treatment.
The inclusion criteria required patients to be over 18 years of age, with histologically proven colorectal cancer to the liver, who were chemotherapy-naive for their metastatic disease, who had at least one measurable liver metastasis >1 cm in size (modified RECIST criteria), with liver-dominant disease (defined as ≥80 % tumor body burden confined to the liver) but less than 60 % liver replacement by tumor, patent main portal vein, Easter Cooperative Oncology Group (ECOG) performance status score of ≤2 and life expectancy of >3 months. They were also required to have adequate hematologic function as evidenced by absolute neutrophil count (ANC)≥1.5 × 109/l, platelets≥75 × 109/l, and international normalized ratio (INR) ≤1.3* (*If a patient was on anticoagulants, they had to be able to stop medication temporarily prior to transarterial chemoembolization (TACE) and have INR ≤1.3 at the time of the procedure.) Adequate liver function for study eligibility was defined by total bilirubin ≤2.0 mg/dl, alanine aminotransferase, aspartate aminotransferase is more than five times upper limit of normal, and albumin≥2.5 g/dl; serum creatinine ≤2.0 mg/dl served as the criterion for acceptable renal function.
Exclusion criteria included patient eligibile for curative treatment (i.e., resection or radiofrequency ablation) and who did not fit inclusion criteria defined above. Note: Resectability was defined by a single tumor <5 cm with adequate liver function (total bilirubin ≤2.0 mg/dl) and the absence of other contravening factors such as more than six tumors, proximity to blood vessels, presence of hepatic-pulmonary shunting, or patients with poor performance (ECOG > 2). Also excluded were patients with history of serious allergy to contrast media that cannot be managed with standard care (e.g., steroids), which would contraindicate magnetic resonance imaging or computed tomography (CT).
The treatment schema involved standard chemotherapy administration on day 0 and day 14, with delivery of DEBIRI being performed on day 7 and day 21 (Table 1). The FOLFOX6-modified dosing was used, with the oxaliplatin dose at 85 mg/m2. 8 The use of bevacizumab was left to the discretion of the treating medical oncologist based on potential contraindications (e.g., intact primary tumor with history of bleeding, recent surgery, cardiovascular issues, etc.). Given the lack of true robust clinical benefit in recent clinical trials, the decision on bevacizumab use was not felt to affect the primary endpoint of the study.
The technique of DEBIRI treatment has been described in detail in our prior studies.11,12 In short, it is performed through a femoral or axillary artery puncture and after appropriate anatomic identification of the right and left hepatic arteries, one vial of beads are eluted with the desired amount of irinotecan chemotherapy. Treatment was performed in a lobar approach, based on the extent and distribution of the disease with most treatments being performed in the outpatient setting. A technical DEBIRI success was defined as the ability to deliver at least 75 % of the planned dose (i.e., at least 75 mg of irinotecan-loaded beads). The number of treatments was determined by the physician after reevaluation with imaging following the four cycles of FOLFOX and two DEBIRI treatments (Table 1) based on the degree of response, tolerance to combination therapy, and quality of life. Hepatic arterial anatomy plays little role in the ability to deliver DEBIRI, given that the large-sized beads so not lead to retrograde flow and allow for accessory or replace arterial anatomy to be treated effectively.
The device used to deliver the irinotecan in this DEBIRI study is an n-Fil sulfonate-modified hydrogel spherical device. The unloaded LC Bead has an FDA 510 k clearance as a Class II embolic device, and is CE mark approved as a drug delivery embolization system, loadable with irinotecan for the treatment of liver metastases of colorectal cancer. DEBIRI was loaded with irinotecan at 50 mg/ml for a total dose of 100 mg/vial. The dosage delivered was defined as the amount of irinotecan given at one DEBIRI administration. The total hepatic exposure was defined as the total sum of irinotecan that was delivered to the patient’s entire liver over the full course of treatment.
DEBIRI is intended as a combination therapy for the treatment of liver metastases arising from colorectal cancer by TACE. The primary function of the device is to embolize the arteries feeding the tumor site, which cause nutrient and oxygen starvation of the tumor and thereby induce necrosis in the tumor tissue. The secondary function is to deliver irinotecan in a controlled manner to the site of the tumors. These functions combine to significantly enhance the cytotoxicity of irinotecan to the tumor and potentially reduce the systemic toxicity compared to intravenous chemotherapy.
Pharmacokinetic evaluation was performed by KAR Bioanalytical (Kalamazoo, MI, 49001) through a quantitative determination of irinotecan in human plasma with lithium heparin. Irinotecan was isolated from human plasma using acidified acetonitrile to precipitate the plasma proteins followed by centrifugation and LC-MS/MS analysis. Blank control human plasma (heparin) was spiked with irinotecan in acidified acetonitrile so that a linear range of quantitation from 0.500 to 500 ng/ml was achieved and was spiked with SN-38 in acidified acetonitrile so that a linear range of quantitation from 0.250 to 50.0 ng/ml was achieved for a 200 μl sample size. Initially, irinotecan to internal standard peak height ratios were fitted to a linear, 1/x 2-weighted regression curve, and SN-38 to internal standard peak height ratios were fitted to a linear, 1/x-weighted regression curve for quantifying quality control samples. Subsequently, irinotecan peak heights were fitted to a linear, 1/x 2-weighted regression curve.
All adverse events were recorded per standards and terminology set forth by the Cancer Therapy Evaluation Program Common Terminology Criteria for Adverse Events, version 3.0. Previous published studies have demonstrated DEBIRI safety as well as efficacy with improved overall survival.12
Follow-up assessments included a tri-phase CT scan of the liver within at least 1–2 months from treatment completion to evaluate the enhancement pattern of the target lesion and tumor response rates measured according to modified RECIST16 criteria. Follow-up assessment was then performed at 3-month intervals for the first year and every 6 months for the second year. Hepatic progression-free survival was defined by liver-only progression of disease, whereas extra-hepatic progression-free survival was defined by the progression outside of the liver (e.g., lung, peritoneum, bone) during follow-up. Surgical respectability was reassessed after each set of four cycles of systemic chemotherapy (i.e., after 4th, 8th, and 12th cycle; Table 1). Decision for surgical resection was made by the treating surgeon based on the established criteria for resectability as reported.17
A central assessment of tumor response was performed for all patients by the principal investigator (PI) at University of Louisville. When there was a discrepancy, the registry PI and the site PI reviewed again for concurrent agreement. Statistical analysis of data was done using JMP 8.0.
Results
Of the 11 eligible patients identified, ten were treated on this prospective Phase I study. The pretreatment patient characteristics are summarized in Table 2. Two patients had a distant prior history of other cancers. A number of five patients who had synchronous colorectal metastasis were initiated into their treatment with a colon or rectal primary in place. There was an even distribution of patients who had liver-only disease and patients who had liver-predominant disease, with limited small-volume disease either in the lung or peritoneum. All ten patients received a full dose of modified FOLFOX 6, with six patients also receiving bevacizumab during their treatment schedule. All patients underwent successful hepatic arterial therapy.
The median total number of liver lesions per patient was four (range 3–25), with median percentage of liver involvement being 35 % (range 25–50). The median size of the target lesions was 11.5 cm (range 6.4–22.1 cm) For the hepatic arterial DEBIRI therapy, five patients underwent two treatments, two patients underwent three treatments, and three patients underwent four treatments, based on their disease distribution and initial response and tolerance. Most common location for treatment was the right lobe, with the level of angiographic bead infusion being a lobar infusion of DEBIRI beads in 100 %. The degree of occlusion following DEBIRI was complete in five procedures, near-complete in another five procedures, and partial in 18 procedures. The median dose delivered was 100 mg/lobe with a range of 40–100 mg (Table 3). The overall length of stay was 23 h, with a single patient requiring an additional hospital stay. The maximum technical dose of DEBIRI was determined by both extent of liver parenchyma to be treated and degree of stasis following last treatment. All patients were able to receive full dose to the right lobe at least two times without technical dose limitation. Systemic exposure was consistently very low and therefore not a factor in determining the extent of maximum dose for any of the patients.
There were six severe adverse events (SAEs) and one device-related SAE, which was a single grade III prolonged increase in blood pressure requiring an additional day in the hospital. This patient had underlying three-drug hypertension, which was not successfully controlled on postprocedure day 1 thereby needing the extra stay. The rest of the SAEs were not device-related and are summarized as follows: The first was a patient who was not on bevacizumab and developed stroke-type symptoms following her 6th cycle of FOLFOX chemotherapy. The second patient was admitted for 23 h for profound dehydration following his 8th cycle of FOLFOX-based chemotherapy. The third presented after her second cycle of chemotherapy and was admitted for dehydration. The fourth was admitted for 2 days for dehydration after the 6th dose of chemotherapy. The fifth was admitted for abdominal pain for 2 days after her first dose of chemotherapy. The sixth was a patient who had his rectal primary in place and, after his 4th dose of chemotherapy, presented with symptoms of obstruction and was admitted for flexible sigmoidoscopy and rectal stenting. We saw no maximum systemic tolerated dose with DEBIRI given the limited device related SAE’s, but that the most common maximum dose of DEIBIRI was decided by the technical ability to deliver a dose as described above.
There were a total of 99 adverse events in these ten patients while they were on active treatment of FOLFOX and DEBIRI. The most common DEBIRI adverse events were hypertension (80 %), abdominal pain (40 %), and nausea (40 %), all of which were grades 1–2 in severity except for the one SAE described above. The most common chemotherapy adverse events were hematologic toxicity (90 %), neurotoxicity (60 %), fatigue (60 %), and one case of alopecia (10 %). All were of grades 1–2 in severity, except for specific cases described above.
No dose reduction modifications required during the patients treatments and no unresolved adverse events were identified during the patients’ active course of treatment with chemotherapy and DEBIRI. There were two minor treatment delays of 2 weeks each due to cytopenias. The first was a 3-week delay in her chemotherapy regimen, following resumption of her chemotherapy, after undergoing complete hepatic resection of all disease. She was restarted on her FOLFOX (cycle 5) and had to have a dose delay because of an ANC of 1350, Hgb = 8.5 g/dl, and PLT = 72 k/ul for additional 2 weeks in order to receive cycle 6. Patient blood counts recovered by week 4 and she was able to resume chemotherapy. The second patient had their chemotherapy held in between cycle 7 and cycle 8 because of an ANC of 630, which required a dose delay of 2 weeks.
Systemic exposure of both irinotecan and its active metabolite SN-38 were evaluated after each DEBIRI treatment (Table 4). There was an obvious peak exposure at 1 h and a fairly rapid resolution of that exposure at 4 h, with essentially undetectable levels at 24 h (Fig. 1a and b). There were no irinotecan-related systemic side effects seen in these ten patients evaluated. Regardless of the number of bead treatments that were administered, this rapid resolution was consistent whether a patient was having their first bead treatment or their fourth bead treatment, who would be a patient who had received at least seven cycles of systemic chemotherapy using this protocol. In comparison with other reported pharmacokinetic studies of systemic irinotecan, the use of DEBIRI demonstrated significant decrease in overall systemic exposure to CPT-11 and Sn-38.
Four patients became eligible for resection following either resolution of their extrahepatic disease or significant response of their bilobar hepatic disease (Table 5). One patient required a portal vein embolization, but all of these four patients were able to undergo major resections (two right hepatectomy, one extended right hepatectomy, one left hepatectomy with microwave ablation of right lobe lesions and extended left colectomy). One patient developed transient hepatic insufficiency after right hepatectomy, which required extended hospitalization for 16 days. The remaining three patients all tolerated surgical resection without significant adverse events. Pathologic response rates in the resected specimens were greater than 95 % in all patients, with significant necrosis and embolic material seen deep within the tumor bead, and minimal embolic material seen in nontumor-bearing liver. All nontumor-bearing liver demonstrated inflammation, but none of them demonstrated any pathologic evidence of steatohepatitis.
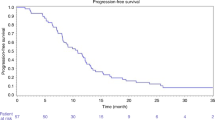
In an evaluation of liver-specific response rates, the 3-, 6-, and 9-month response rates were 100 %, with the 12-month rate being 90 %. Overall, response rates were 100 % at 3 months, 80 % at 6 months, 60 % at 9 months, and 50 % at 12 months mostly because of ongoing progression in either the lungs or the peritoneum. Median overall survival was 15.2 months (range 6.5–45.5 months).
Discussion
Colorectal carcinoma (CRC) is the second most common cause of cancer-related death in developed countries.18 The combinations of irinotecan (CPT-11) or oxaliplatin with FU/LV have demonstrated increased efficacy compared with FU/LV alone in randomized studies.6,19,20 These data have established that in metastatic colorectal cancer, a more active first line treatment can be also more effective in terms of improved progression-free survival and overall survival.21 In this report, we present the first study utilizing this four-drug regimen, in which the irinotecan is delivered directly to the organ with the largest tumor burden (i.e., the liver, with >80 % overall tumor burden). This therapy has the advantage of allowing for the combination of continuous full-dose systemic therapy with hepatic arterial directed therapy, so that the additive or synergistic activity can occur at the site of the greatest tumor burden, but without overlapping systemic toxicity. This pilot study has confirmed minimal to nonexistent systemic irinotecan exposure, with significant response rates, higher than usual conversion to resection with minimal hepatic damage following therapy.
Recent reports have demonstrated the safety11 and efficacy,12 and responsiveness of DEBIRI as a single modality in chemorefractory patients13 with liver-dominant metastatic colorectal cancer. This is the first study to demonstrate its pharmacokinetics, clinical tolerability and activity in combination with concurrent systemic chemotherapy in the first line setting. In addition, this data demonstrates the feasibility in liver-predominant, first line metastatic colorectal cancer in combination with first-line systemic chemotherapy. This combination therapy does not require a change in systemic chemotherapy dosing or timing since the DEBIRI treatment is performed during the off week. Similarly, the technique for DEBIRI is simplified by using only a lobar infusion to minimize toxicity22 and optimize drug delivery.
In comparison, to know pharmacokinetics of CPT-11 and its active metabolite SN-38, the use of DEBIRI via hepatic arterial delivery leads to significant reduction of systemic exposure of both CPT-11 and SN-38 (Table 6). One of the initial studies by de Jonge et al.23 demonstrated that with similar systemic dosing of CPT-11, the peak CPT-11 level was 3842.7 ng/ml and SN-38 was 29.42 ng/ml. In a similar study by van Riel et al.,24 the pharmacokinetic exposure after 5-day infusion of CPT-11 with a similar cumulative dosage was a level of CPT-11 of 76.27 ng/ml and SN-38 of 5.57 ng/ml. In a direct comparison of systemic CPT-11 to hepatic intra-arterial infusion of CPT-11, Mambrini et al.25 demonstrated systemic exposures which were consistently higher than those seen in our study and consistent with the de Jonge data (Table 6).
A study by the Groupe Coope’rateur Multidisciplinaire en Oncologie19 suggested that the exposure of metastatic colorectal cancer patients to both FOLFOX and FOLFIRI, irrespective of their sequence, is associated with the most optimal survival. Moreover, a pooled analysis of seven phase III trials demonstrated that patients who received all the three active drugs in the course of their disease achieved the longest overall survival.6 This analysis also shows that, in a sequential strategy, not all patients who progress after first-line chemotherapy are able to receive second-line treatment (50–80 %). A way to further improve the outcome of metastatic colorectal cancer patients could be to administer a first-line regimen containing all the three active agents (oxaliplatin, irinotecan, and FU/LV). There is available data suggesting that such a regimen can be more active than a standard two-drug combination and could also increase the postchemotherapy resection rate of metastases and, therefore, the long-term control of disease.10 In fact, studies conducted with oxaliplatin, and FU-based regimens4,5 and, more recently, also with other combinations, indicate that an active first-line chemotherapy in initially unresectable patients can allow a radical resection of metastatic disease and 20 % to 40 % of these resected patients are long-term survivors. In particular, an analysis conducted by Folpercht et al.10 demonstrates a correlation between the response rate to chemotherapy and the postchemotherapy radical resection rate of metastases.
The results presented here demonstrate the safety and feasibility of administering this four-drug regimen with significantly less systemic toxicity, and with the hypothetical advantage of targeted hepatic therapy, thus widening the therapeutic window. Similarly, the pharmacokinetic data confirm the minimal systemic exposure of both irinotecan and its active metabolite SN-38 when utilizing hepatic arterial drug-eluting bead therapy. This data confirms the concept of point-directed chemotherapy without systemic exposure, which thus allow for higher drug concentration to be delivered, higher drug retention into the tumor, with increased therapeutic efficacy and thus better overall compliance due to lower side effects.
Continued evaluation of this FOLFOXDEBIRI, regimen is necessary and is ongoing with the recent opening of the randomized phase II trial of FOLFOX±bevacizumab versus FOLFOX±bevacizumab with DEBIRI (ClinicalTrials.gov identifier: NCT00932438).
Conclusion
Concomitant DEBIRI and FOLFOX±bevacizumab is safe, with a minimal adverse event rate, no dose-limiting toxicities, and enhanced overall response rate. Continued demands for liver-dominant trial design and ongoing randomized evaluation of this regimen should further confirm these initial results.
References
Welch JP, Donaldson GA. The clinical correlation of an autopsy study of recurrent colorectal cancer. Ann Surg 1979; 189:496–502.
Silverberg E. Cancer statistics, 1977. CA Cancer J Clin 1977; 27:26–41.
Scheele J, Stangl R, Altendorf-Hofmann A et al. Indicators of prognosis after hepatic resection for colorectal secondaries. Surgery 1991; 110:13–29.
Adam R, Avisar E, Ariche A et al. Five-year survival following hepatic resection after neoadjuvant therapy for nonresectable colorectal. Ann Surg Oncol 2001; 8:347–353.
Bismuth H, Adam R. Reduction of nonresectable liver metastasis from colorectal cancer after oxaliplatin chemotherapy. Semin Oncol 1998; 25:40–46.
Grothey A, Sargent D, Goldberg RM et al. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil–leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol 2004; 22:1209–1214.
Goldberg RM, Sargent DJ, Morton RF et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 2004; 22:23–30.
Cheeseman SL, Joel SP, Chester JD et al. A ‘modified de Gramont’ regimen of fluorouracil, alone and with oxaliplatin, for advanced colorectal cancer. Br J Cancer 2002; 87:393–399.
Colucci G, Gebbia V, Paoletti G et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell’Italia Meridionale. J Clin Oncol 2005; 23:4866–4875.
Falcone A, Ricci S, Brunetti I et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol 2007; 25:1670–1676.
Martin RC, Joshi J, Robbins K et al. Transarterial chemoembolization of metastatic colorectal carcinoma with drug-eluting beads, irinotecan (DEBIRI): multi-institutional registry. J Oncol 2009; 2009:539795.
Martin RC, Robbins K, Tomalty D et al. Transarterial chemoembolisation (TACE) using irinotecan-loaded beads for the treatment of unresectable metastases to the liver in patients with colorectal cancer: an interim report. World J Surg Oncol 2009; 7:80.
Martin RC, Joshi J, Robbins K et al. Hepatic intra-arterial injection of drug-eluting bead, irinotecan (DEBIRI) in unresectable colorectal liver metastases refractory to systemic chemotherapy: results of multi-institutional study. Ann Surg Oncol 2011; 18:192–198.
Bower M, Metzger T, Robbins K et al. Surgical downstaging and neo-adjuvant therapy in metastatic colorectal carcinoma with irinotecan drug-eluting beads: a multi-institutional study. HPB (Oxford) 2010; 12:31–36.
Brown RE, Bower MR, Metzger TL et al. Hepatectomy after hepatic arterial therapy with either yttrium-90 or drug-eluting bead chemotherapy: is it safe? HPB (Oxford) 2011; 13:91–95.
Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45:228–247.
Vauthey JN, Choti MA, Helton WS. AHPBA/SSO/SSAT consensus conference on hepatic colorectal metastases: rationale and overview of the conference. January 25, 2006. Ann Surg Oncol 2006; 13:1259–1260.
Jemal A, Thomas A, Murray T et al. Cancer statistics, 2002. CA Cancer J Clin 2002; 52:23–47.
Folprecht G, Grothey A, Alberts S et al. Neoadjuvant treatment of unresectable colorectal liver metastases: correlation between tumour response and resection rates. Ann Oncol 2005; 16:1311–1319.
Hebbar M, Tournigand C, Lledo G et al. Phase II trial alternating FOLFOX-6 and FOLFIRI regimens in second-line therapy of patients with metastatic colorectal cancer (FIREFOX study). Cancer Invest 2006; 24:154–159.
Buyse M, Thirion P, Carlson RW et al. Relation between tumour response to first-line chemotherapy and survival in advanced colorectal cancer: a meta-analysis. Meta-analysis group in cancer. Lancet 2000; 356:373–378.
Martin RC, Howard J, Tomalty D et al. Toxicity of irinotecan-eluting beads in the treatment of hepatic malignancies: results of a multi-institutional registry. Cardiovasc Intervent Radiol 2010; 33:960–966.
de Jonge MJ, Verweij J, de BP et al. Pharmacokinetic, metabolic, and pharmacodynamic profiles in a dose-escalating study of irinotecan and cisplatin. J Clin Oncol 2000; 18:195–203.
van Riel JM, van Groeningen CJ, Kedde MA et al. Continuous administration of irinotecan by hepatic arterial infusion: a phase I and pharmacokinetic study. Clin Cancer Res 2002; 8:405–412.
Mambrini A, Di Paolo A, Pacetti P, Muttini MP, Orlandi M, Danesi R, Fiorentini G, Cantore M. Pharmacokinetics of irinotecan: comparison of intravenous and intraarterial administration in patients with liver metastases. J Clin Oncol 2008; 26:2234.
Conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martin, R.C.G., Scoggins, C.R., Tomalty, D. et al. Irinotecan Drug-Eluting Beads in the Treatment of Chemo-Naive Unresectable Colorectal Liver Metastasis with Concomitant Systemic Fluorouracil and Oxaliplatin: Results of Pharmacokinetics and Phase I Trial. J Gastrointest Surg 16, 1531–1538 (2012). https://doi.org/10.1007/s11605-012-1892-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-012-1892-8