Abstract
Purpose
The nonthermal irreversible electroporation (NTIRE) is a novel nonthermal tissue ablation technique by local application of high-voltage current within microseconds leading to a delayed apoptosis. The purpose of this experimental study was the first angiographic evaluation of the acute damage of renal vascular structure in NTIRE.
Methods
Results of conventional dynamic digital substraction angiography (DSA) and visualization of the terminal vascular bed of renal parenchyma by high-resolution X-ray in mammography technique were evaluated before, during, and after NTIRE of three isolated perfused porcine ex vivo kidneys.
Results
In the dedicated investigation, no acute vascular destruction of the renal parenchyma and no dysfunction of the kidney perfusion model were observed during or after NTIRE. Conspicuous were concentric wave-like fluctuations of the DSA contrast agent simultaneous to the NTIRE pulses resulting from NTIRE pulse shock wave.
Conclusion
The NTIRE offers an ablation method with no acute collateral vascular damage in angiographic evaluation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nonthermal irreversible electroporation (NTIRE) is a novel nonthermal tissue ablation technique that applies high-voltage and high-current electrical pulses on the microsecond timescale with inserted needle-like electrodes to induce irreversible permeabilization of the cell membrane (nanopores) with consequent cell death by loss of homeostasis within approximately 4 (±3) days. Although the exact mechanism of electroporation, reversible as well as irreversible, is not completely understood, it is known that NTIRE alters in vivo cells on molecular level via induced high-electric field transmembrane voltage that causes an electrical breakdown of the dielectric lipid bilayer. NTIRE does not alter the extracellular matrix and does not cause protein denaturation or other side effects that are associated with thermal ablation modalities. Hence, anatomical borders and structures-like vessels and nerves as well as the integrity of an organ are safe [1]. Recent studies have shown a multidisciplinary potential in surgical oncology and ablation of benign lesions. It has been used in different preclinical models of tissue ablation of organs, such as kidney, prostate, liver, heart, pancreas, brain, lung, dermis, and vessels [2–12]. In contrast to current thermal ablative techniques, there is no reduction of effectiveness by vascularization (“cold- or heat-sink effect”) [13] and no collateral damage to anatomical borders. Several investigations have demonstrated the persisting intactness of major vessels adjoining to or lying in the ablation zone as well as the necrosis and thrombosis of small vessels in the ablation zone [1, 11]. So, the possible protection of the urine collecting system, such as the renal pelvis and ureter, as well as a nephron-sparing effect by sharply bounded ablation zone is postulated by using NTIRE in kidney [2, 6].
To date, there is no sufficient real-time imaging method that is able to correlate the ablation area during or immediately after NTIRE to the tumor extension [13–15]. Experimental studies have demonstrated the confirmation of a hyperechoic areal in real-time ultrasound respectively a hypodense areal in real-time computer tomography (CT) immediately after the NTIRE around the probe as well as a T2-hyperintense areal in MRT 1 day after NTIRE [11, 16]. These areals were correlated with the histopathological ablation zones in delayed exploration of in vivo target tissues and are explained as acute edema of the ablated area as a sign of local inflammatory tissue reaction on energy application [13, 16]. For follow-up after NTIRE, conventional imaging methods, such as CT or MRT, are reliable methods to investigate tumor ablation by showing necrosis, scar formation, tumor size reduction, and absence of enhancement [17]. Despite of all that, only postresection histological analysis in a closed interval of 2–4 weeks after NTIRE is able to prove complete tumor ablation.
With our investigations, we focused on NTIRE as a novel potential ablation modality for renal cell carcinoma (RCC). First results of clinical intraoperative trials have shown the possibility of safe application of NTIRE to the kidney using ECG-synchronization and general anesthesia with muscle relaxation [18, 19]. Pech et al. [18] performed an intraoperative ultrasound-guided NTIRE of localized RCC (tumor size 20–39 mm) in six patients scheduled for curative open lumbar resection (four partial and two total nephrectomies). For all patients a NanoKnife™ system with bipolar single probe and 90 pulses up to 50 A and 3000 V by a NanoKnife™ generator (AngioDynamics® Inc., NY, USA) was used. They observed no acute side effects during the peri- and postoperative (12 weeks) periods by measuring ECG, blood pressure, central hemodynamic values, standard blood values, acid-base values, and respiratory values. Thomson et al. [17] investigated ECG synchronized IRE in 38 humans with advanced malignancy of the liver (n = 25), kidney (n = 7), and lung (n = 3) and a total of 69 separate tumors (average 46 cm³) that where unresponsive for alternative treatment. They observed a complete target tumor ablation verified by CT in 66% (46/69) without NTIRE-related adverse events.
The kidney offers a favorable target for tumor ablation because of its location for percutaneous access and its condition of severe vascularized parenchyma, which is easy to monitor by routine imaging procedures as well as blood and urine values. The therapy “gold standard” of small localized RCC (T1-2 TNM 2010 UICC) is the nephron-sparing surgery by partial tumor nephrectomy [20]. One basis of the nephron-sparing therapy to reduce the risk of renal failure is the maximum protection of the local vascular system and prevention of ischemia. For inoperable cases and patients with small, multilocular, or bilateral renal tumors as well as solitary kidney, the 2010 EAU guidelines recommended cryoablation and radiofrequency ablation (RFA) as therapeutic alternatives [20]. These thermal ablation techniques cause acute destruction of the vascular system and tissue, in both of the target tissue as well as the adjacent tissue in terms of collateral damage.
This experimental study was the first radiological evaluation of the acute vascular damage of renal parenchyma by NTIRE in relation to thermal ablation techniques.
Material and Methods
Selection and Preparation of the Kidneys
Three kidneys were obtained from three freshly slaughtered pigs (7 min) in warm ischemia. Only kidneys from pigs for slaughter of an accredited abattoire (estate Gut Glüsig GmbH, Germany) were taken after termination. The healthiness of the pigs (7 ± 1-month-old and weighed 120 ± 10 kg) was examined by veterinary. The preparations of the isolated kidneys were performed by two experienced urologists according to the technique of kidney transplantation.
The renal hilum was prepared immediately after organ explantation. The kidneys were directly intra-arterially perfused with 12.500 IE of heparin (0.5 ml of Heparin-Natrium-12.500-ratiopharm®, Ratiopharm, Germany) by a cannula to avoid vascular clotting (after 3 min).
The renal artery and renal vein were intubated with flexible tube sections of infusion elongation (Heidelberg Extension Tubing®, Braun, Germany) and fixed by ligature (Marlin® 1, Catgut, Germany). To avoid air embolization, these preparations were performed in a water bath of 4°C NaCl 0.9%. While unhinging the kidneys from adipose capsule, we researched pathological alterations to exclude any impact.
After 10 min, we intra-arterially perfused the kidneys with 500 ml of cold (4°C) NaCl 0.9% (Intrafix® Primeline Braun, Germany) with a pressure of 100 ± 5 cm H2O by pressure infusion cuff (SafePress 1000 CC®, Dahlhausen, Germany), until nonbloody solution drain off the renal vein. The proximal ureter was intubated with a percutaneous nephrostomy balloon-catheter 10 Charr. (Uromed®, Germany) blocked with 2 ml in the renal pelvis. Consequently, we stored the kidneys in NaCl 0.9% at 4°C until performing NTIRE.
Perfusion Model of the Isolated Kidney
To simulate a physiological temperature, the kidneys were completely lying in NaCl 0.9% (35°C) during NTIRE and digital substraction angiography (DSA) (50 min after isolation) and perfused continuously with NaCl 0.9% (35°C) through the renal arteries at 100 ml/min and a pressure between 100 ± 10 cm H2O (measured by water column) using a commercial perfusion pump for percutaneous nephrolitholapaxy (Uropump®, Storz, Germany) and an air chamber. To exclude any extravasation and control complete perfusion of the organ, we applied indigo blue colorant (MonicoSpa, Spain). Venous and ureteral outflow were collected and measured separately (Fig. 1). A continuous, nearly physiological perfusion of the kidneys was feasible. We observed no relevant extravasation due to any leakage and the perfusion of the kidneys seemed to be complete by changing color while flushing with NaCl 0.9% and indigo blue colorant. Venous and ureteral outflow was constant at approximately 9:1 while constant pressure 100 ± 10 cm H2O and constant perfusion 100 ± 10 ml/min.
Nonthermal Irreversible Electroporation
During the continuous perfusion of the isolated porcine kidneys, we performed NTIRE. Therefore, each of the three kidneys was punctured manually in the lower, middle, and upper part (total amount: nine ablation zones) per one NTIRE electrode (5 Charr. Resp. 1.6 mm). For all NTIRE applications, a NanoKnife™ Electroporator (AngioDyanmics® Inc.) with a bipolar single-needle electrode (NanoKnife™ bipolar probe 15 cm, AngioDyanmics® Inc.) was used. In each part, 90 pulses per minute were given with 2700 V approximately 22 A and 70 ms. As specified by the manufacturer, the calculated ablation zone is a prolate spheroid of 30 × 15 mm2. The total energy averaged 375 kJ per ablation zone resp. approximately 1 mJ per kidney. Each kidney was examined by two independent and experienced urologists in consensus. Each NTIRE procedure was executed sufficiently, and no undelivered pulses were documented. During NTIRE, we observed no change of the perfusion performance.
Digital Substraction Angiography
To visualize the dynamic perfusion, we performed DSA (AXIOM Artis FA®, Siemens, Germany) of the perfused kidneys with three pictures per second. For each DSA series, 20 ml of iodine contrast agent (Imeron® 300, Bracco Imaging, Italy) was injected to the perfusion system. Each DSA series (Figs. 2, 3) was reviewed by two independent and experienced interventional radiologists and two independent and experienced urologists in consensus.
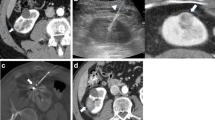
Display detail of the surrounding area of the single-needle bipolar probe during digital substraction angiography (DSA) while performing NTIRE to the lower part of the isolated perfused porcine kidney. The open black arrows show the fluctuation of the X-ray contrast agent based on the apex and the basis of the nonisolated lower pole of the NTIRE single-needle electrode (see Fig. 2)
Marker and Digital Angiography in Mammography Technique (Static Angiography)
Marking the exact NTIRE probe position in the static angiography X-ray images in mammography technique (low-kV exposure technique, high resolution), we used self-made marker (5 Charr.) consisting of a 16 G venous in-dwelling catheter (BD Venflon™ Pro Safety, Becton Dickenson, USA). We used the tube fragment of approximately 30 mm and fixed a 10-mm piece of copper wire in the middle of the lumen. The ends of the tube were closed with the correspondent mandarin (10 mm). Each one marker was placed in Seldinger technique into one of the three puncture channels of each kidney after each NTIRE application by using a slim synthetic trocar of a 5 Charr. suprapubic catheter for children (EasyCyst®, Rüsch, Germany).
Directly afterwards each kidney was perfused with NaCl 0.9% (4°C) again until the iodine contrast agent for DSA was cleared out. Accordingly the kidneys were perfused with barium sulphate contrast agent (Barilux® HD, Sanochemia, Switzerland) into the renal artery until draining it off the renal vein. Then, the arterial, venous, and ureteral tubes were closed to keep the intravascular and nondiffusible contrast agent inside. Subsequently digital static angiography was performed by mammography technique with 50 cm, 24 kV (low-kV exposure technique, high resolution) and 80 mAs (Mammomat 3000®, Siemens, Germany). Each X-ray image (whole kidney and its 5-mm coronary transactions; Figs. 4, 5) was observed by two independent and experienced interventional radiologists and two independent and experienced urologists in consensus.
Images of the half-split kidney (left) and correspondent digital static angiography per high-resolution X-ray imaging in low-kV exposure mammography technique (right). Open arrows show a small pre-existing cortical cyst. Closed arrows show the metal markers set to the NTIRE electrode channels (3 per kidney)
Digital static angiography per high-resolution X-ray imaging in low-kV exposure mammography technique of the coronary kidney transactions display detailed the renal vascular system around the metal markers (upper pole to the left, central zone in the middle, lower pole to the right). Each metal marker shows one of the NTIRE puncturing channels
Results
Digital Substraction Angiography
Before NTIRE, we observed regular and physiological perfusion with flooding and complete draining off the vascular system of the renal parenchyma without any perfusion gaps. During (Figs. 2, 3) and after (Figs. 4, 5) NTIRE, we observed no relevant changes, such as extravasations, perfusion gaps, open areas, accumulations, or stases in the renal parenchyma. Conspicuous was a concentric wavelike fluctuation of the contrast agent, each based on the apex and basis of the nonisolated lower pole of the NTIRE single-needle bipolar probe, which appeared simultaneous to the NTIRE pulses (90/min), expanded around 1 cm and cleared away completely during perfusion (Fig. 3).
Overall it has to be noted that just major renal vessels, especially of the renal medulla, are detailed visible in DSA, whereas the vascular system of the renal cortex between the renal medulla and the renal capsule seems to be spared (Fig. 2). Because of that, in the following, these visible major vessels in DSA are defined as macrovascular system of the kidney.
Digital Angiography in Mammography Technique (Static Angiography)
The correlation of the NTIRE ablation zone to the angiography images was feasible by marking the NTIRE puncture channels per metal marker (Figs. 4, 5, closed arrows). The contrast agent stayed characteristically intraluminal of the complete organ. In correlation to the marked ablation zones, we observed no extravasation and no disruption of the terminal vascular bed of renal cortical parenchyma, such as gaps, open areas, or accumulations—neither around the seeds nor in the distant parenchyma (Figs. 4, 5). Additional to the DSA, also small vessels of the renal cortex are displayed straight to the renal capsule and can be well distinguished from each other (Fig. 5). In the following these small vessels, which are not detailed visible in DSA, are defined as microvascular system of the kidney.
Discussion
The principle of NTIRE is the local application of high-voltage current within microseconds with a delayed parenchyma ablation after 4 ± 1 days without destruction of the tissue scaffolding and the major vascular system [1, 21], preserving organ structure and the demonstrated recovery of normal tissue with regenerative capacities [5]. Because of supposing no adverse effects, such as collateral damage of the surrounded tissue and vascular system as well as no fragmentary tumor destruction, NTIRE could be superior to current thermal ablation techniques, such as RFA and cryotherapy in kidney tumors [2, 6]. So far, there has been no radiological examination of the vascular system closed to the NTIRE ablation zone in the acute phase but only histological investigations [1, 21].
This experimental study is the first radiological approach to examine the renal vascular system in the acute phase of NTIRE. Conventional DSA before, during, and after NTIRE as well as digital static angiography per high resolution X-ray imaging in low-kV exposure mammography technique after NTIRE were used in isolated, perfused, porcine, ex vivo kidneys. This enables a qualitative radiological examination of the NTIRE ablation zone closed and surrounded terminal macro- and microvascular bed of renal parenchyma in the acute phase. Referred to its typical anatomy of the renal vascular system, the DSA shows the renal macrovascular system with vasa renales, segmentales, interlobares, and arcuatae of the renal medulla and columns (Fig. 2), whereas the angiography in mammography technique additionally shows the renal microvascular system with vasa corticales, rectae spuriae, and rectae verae of the renal cortex and pyramids (Figs. 4, 5).
By using this ex vivo model of the isolated porcine kidney, it is possible to simulate mechanically a current physiological perfusion of a human in vivo kidney to examine acute mechanical vascular reactions and tissue damage by NTIRE without interfering by kidney movement. Because of the restriction status, using NTIRE to humans just in clinical studies, it was not possible to observe percutaneous NTIRE to human unaffected in vivo kidneys by using DSA and static angiography in mammography technique.
We observed at first a remained vascular perfusion and integrity of the terminal vascular bed of renal parenchyma without any lesions in the ablation zone or the surrounded parenchyma by using contrast agent-based X-ray imaging during and after NTIRE. In contrast to that, previous reports on several energy-based kidney treatment methods in the isolated perfused kidney observed lesions of the terminal vascular bed of renal cortical parenchyma with petechial extravasations and open areas, such as in ESWL, respectively cavern-like thermolesions, such as in HIFU using angiography in mammography technique [22–24]. Similar characteristics were observed for other technologies for minimally invasive ablation of renal masses, such as cryoablation, RFA, and microwave [25].
Previous publications reported a remarkable phenomenon of blood perfusion disruption with consecutive ischemia during in vivo electroporation, referred to as the vascular lock and explained as electrical vasoconstriction reflex or collapse by decreased intravascular pressure of afferent arterioles [26]. Other experimental studies of histological analysis after NTIRE of in vivo porcine liver demonstrated intact major vascular structures and necrotic small vessels with organized intraluminal fibrin thrombi in the ablated area 24 hours after NTIRE [1, 11]. Histopathologic analyses of renal tumor resections immediately after NTIRE showed the preservation of the vasculature in the electroporated tissue and the urine collecting system [2, 6, 18].
Incidentally we discovered a conspicuous phenomenon of concentric wavelike fluctuation of the contrast agent simultaneous to the NTIRE pulses (90/min). Each was based on the apex and basis of the NTIRE single-needle bipolar probe with an extension of 1 cm and cleared away completely during perfusion. These contrast agent waves followed no macroscopic visible vascular pattern, if procurable to judge, what may be based on the limited resolution of the DSA. Given that the microvascular structure after NTIRE is maintained in high-resolution angiography imaging by mammography technique, we exclude any destructions, such as extravasation. In fact compared with barium X-ray contrast agent (mammography technique), iodine X-ray contrast agent (DSA) is diffusible, but the pulsatile contrast agent waves occur and clear away too fast and straightened for any physiological or electrical triggered diffusion into the extravascular space. Thus, we conclude that the phenomenon is based on a pulsatile propagation along the capillaries with a fast draining off, what is not detailed presentable in the DSA and may extends three-dimensional starting from the bipolar probe poles.
We hypothesize that the contrast agent waves result from the electrical pulse shock waves caused by the NTIRE strokes and based on the temporary increased intravascular concentration of the contrast agent. This observation is limited by the difficult demonstration of a rapid recurrent dynamic phenomenon in DSA sequence by means of freezed images. This first described radiological phenomenon in NTIRE could be a part for an approach to develop a perfusion-based and reliable modified real-time monitoring method of the NTIRE ablation zone with additional imaging of the tumor extension by its specific vascularization pattern.
This study is limited by the design of an ex vivo model and the small number of studied kidneys (n = 3) and ablation zones (n = 9). The DSA is a dynamic real-time imaging method but is limited by its low resolution and frame frequency. The static angiography per mammography technique is limited by its nonexisting real-time and dynamic imaging but features a high resolution. Moreover, the study is limited to a conventional visualized qualitative evaluation of the renal vascular system without any quantitative analysis. No correlation to thermal ablation methods has been made by the same investigation method. Furthermore, no histological examination in view of vascular system was performed. The ex vivo model cannot reproduce the dynamic behavior of in vivo capillaries due to high-voltage current, the filtration or absorption of in vivo kidney, and the delayed histological or immunological behaviour to NTIRE. A histological analysis could be limited by artefacts of the nondiffusible barium sulphate contrast agent because of vessel obliteration.
In summary, this study is an additional module to demonstrate the preservation of the vascular system closed to the NTIRE ablation zone. This reveals the possible superiority of the NTIRE versus thermal ablation methods for nephron-sparing therapy to reduce the risk of renal failure. Further experimental and clinical in vivo studies are needed for complete understanding the NTIRE mechanism and developing it to a widely useable treatment modality especially of kidney tumors.
Conclusions
This experimental study is the first radiological approach to examine the acute dynamic vascular perfusion of the isolated perfused porcine kidney by conventional digital substraction angiography (DSA) during NTIRE and the morphology of the terminal vascular bed of the renal parenchyma by digital static angiography per high resolution X-ray imaging in low-kV exposure mammography technique after NTIRE. This study is an additional, radiological module to demonstrate the preservation of the macro- and microvascular system of the whole kidney after NTIRE. In contrast to thermal ablation methods, there seems to be no acute vascular damage by NTIRE. This reveals the possible superiority of the NTIRE versus thermal ablation methods for nephron-sparing therapy to reduce the risk of renal failure. Further experimental and in vivo studies are needed for complete understanding the NTIRE mechanism and using NTIRE for ablation of kidney tumors.
References
Rubinsky B, Onik G, Mikus P (2007) Irreversible electroporation: a new ablation modality—clinical implications. Technol Cancer Res Treat 6(1):37–48
Tracy CR, Kabbani W, Cadeddu JA (2010) Irreversible electroporation (IRE): a novel method for renal tissue ablation. BJU Int. doi:10.1111/j.1464-410X.2010.09797.x
Onik G, Mikus P, Rubinsky B (2007) Irreversible electroporation: implications for prostate ablation. Technol Cancer Res Treat 6(4):295–300
Dupuy DE, Aswad B, Ng T (2011) Irreversible electroporation in a swine lung model. Cardiovasc Intervent Radiol 34:391–395
Sano MB, Neal RE 2nd, Garcia PA, Gerber D, Robertson J, Davalos RV (2010) Towards the creation of decellularized organ constructs using irreversible electroporation and active mechanical perfusion. Biomed Eng Online 9(1):83
Deodhar A, Monette S, Single GW Jr, Hamilton WC Jr, Thornton R, Maybody M, Coleman JA, Solomon SB (2011) Renal tissue ablation with irreversible electroporation: preliminary results in a porcine model. Urology 77:754–760
Garcia PA, Neal RE, Rossmeisl JH, Davalos RV (2010) Non-thermal irreversible electroporation for deep intracranial disorders. Conf Proc IEEE Eng Med Biol Soc 1:2743–2746
Charpentier KP, Wolf F, Noble L, Winn B, Resnick M, Dupuy DE (2010) Irreversible electroporation of the pancreas in swine: a pilot study. HPB (Oxford) 12(5):348–351
Maor E, Ivorra A, Mitchell JJ, Rubinsky B (2010) Vascular smooth muscle cells ablation with endovascular nonthermal irreversible electroporation. J Vasc Interv Radiol 21(11):1708–1715
Al-Sakere B, André F, Bernat C, Connault E, Opolon P, Davalos RV, Rubinsky B, Mir LM (2007) Tumor ablation with irreversible electroporation. PLoS One 2(11):e1135
Lee EW, Chen C, Prieto VE, Dry SM, Loh CT, Kee ST (2010) Advanced hepatic ablation technique for creating complete cell death: irreversible electroporation. Radiology 255(2):426–433
Hong J, Stewart MT, Cheek DS, Francischelli DE, Kirchhof N (2009) Cardiac ablation via electroporation. Conf Proc IEEE Eng Med Biol Soc 2009:3381–3384
Lee EW, Thai S, Kee ST (2010) Irreversible electroporation: a novel image-guided cancer therapy. Gut Liver 4(Suppl 1):S99–S104
Goldberg SN, Charboneau JW, Dodd GD 3rd, Dupuy DE, Gervais DA, Gillams AR, Kane RA, Lee FT Jr, Livraghi T, McGahan JP, Rhim H, Silverman SG, Solbiati L, Vogl TJ, Wood BJ, International Working Group on Image-Guided Tumor Ablation (2003) Image-guided tumor ablation: proposal for standardization of terms and reporting criteria. Radiology 228(2):335–345
Granot Y, Ivorra A, Maor E, Rubinsky B (2009) In vivo imaging of irreversible electroporation by means of electrical impedance tomography. Phys Med Biol 54(16):4927–4943
Lee EW, Loh CT, Kee ST (2007) Imaging guided percutaneous irreversible electroporation: ultrasound and immunohistological correlation. Technol Cancer Res Treat 6(4):287–294
Thomson KR, Cheung W, Ellis SJ, Park D, Kavnoudias H, Loader-Oliver D, Roberts S, Evans P, Ball C, Haydon A (2011) Investigation of the safety of irreversible electroporation in humans. J Vasc Interv Radiol 22(5):611–621
Pech M, Janitzky A, Wendler JJ, Strang C, Blaschke S, Dudeck O, Ricke J, Liehr UB (2011) Irreversible electroporation of renal cell carcinoma: a first-in-man phase I clinical study. Cardiovasc Intervent Radiol 34:132–138
Ball C, Thomson KR, Kavnoudias H (2010) Irreversible electroporation: a new challenge in “out of operating theater” anesthesia. Anesth Analg 110(5):1305–1309
Ljungberg B, Cowan N, Hanbury DC, Hora M, Kuczyk MA, Merseburger AS, Mulders PFA, Patard JJ, Sinescu IC (2010) Guidelines on Renal Cell Carcinoma. European Association of Urology (EAU). http://www.uroweb.org/. Accessed 25 May 2011
Maor E, Ivorra A, Leor J, Rubinsky B (2007) The effect of irreversible electroporation on blood vessels. Technol Cancer Res Treat 6(4):307–312
Häcker A, Chauhan S, Peters K, Hildenbrand R, Marlinghaus E, Alken P, Michel MS (2005) Multiple high-intensity focused ultrasound probes for kidney-tissue ablation. J Endourol 19(8):1036–1040
Köhrmann KU, Back W, Bensemann J, Florian J, Weber A, Kahmann F, Rassweiler J, Alken P (1994) The isolated perfused kidney of the pig: new model to evaluate shock wave-induced lesions. J Endourol 8(2):105–110
Peters K (2007) Experimetelle Untersuchungen zur nichtinvasiven Gewebeablation durch hochenergetischen fokussierten Ultraschall (HIFU). Dissertation, Tierärztliche Fakultät der LMU München. http://edoc.ub.uni-muenchen.de/6643/1/Peters_Kristina.pdf
Duffey BG, Kyle Anderson J (2010) Current and future technology for minimally invasive ablation of renal cell carcinoma. Indian J Urol 26(3):410–417
Sersa G, Jarm T, Kotnik T, Coer A, Podkrajsek M, Sentjurc M, Miklavcic D, Kadivec M, Kranjc S, Secerov A, Cemazar M (2008) Vascular disrupting action of electroporation and electrochemotherapy with bleomycin in murine sarcoma. Br J Cancer 98(2):388–398
Conflict of interest
The authors declare that they have no conflicts of interest. This study was performed independently of the manufacturer of the devices used.
Author information
Authors and Affiliations
Corresponding author
Additional information
Johann Jakob Wendler and Maciej Pech are contributed equally to this study.
Rights and permissions
About this article
Cite this article
Wendler, J.J., Pech, M., Blaschke, S. et al. Angiography in the Isolated Perfused Kidney: Radiological Evaluation of Vascular Protection in Tissue Ablation by Nonthermal Irreversible Electroporation. Cardiovasc Intervent Radiol 35, 383–390 (2012). https://doi.org/10.1007/s00270-011-0187-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-011-0187-x