Abstract
Late complications of thrombosis of the deep veins in the region between the popliteal vein termination and the confluence of the common iliac veins and inferior vena cava (suprapopliteal deep-vein thrombosis) are common and often unrecognized by those responsible for the initial management. Pharmacomechanical-assisted clearance of the thrombus at the time of first presentation provides the best opportunity for complete recovery with preservation of normal venous valve function and avoidance of recurrent deep-vein thrombosis and postthrombotic syndrome. Recent interventional radiology methods provide for rapid and complete thrombolysis even in some patients in whom thrombolysis was previously considered contraindicated. This review describes the methods, safety, and efficacy of acute interventional treatment of suprapopliteal deep-vein thrombosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thrombosis of the deep veins in the region between the popliteal vein termination and the confluence of the common iliac veins and inferior vena cava (suprapopliteal deep-vein thrombosis [DVT]) is a condition that is usually treated in the acute phase by anticoagulation with bed rest and compression stockings. Late complications of this treatment are common and often unrecognized by those responsible for the initial management. As a result, patients are left with significant morbidity from postthrombotic complications that might have been avoided with pharmacomechanical-assisted clearance of the thrombus at the time of first presentation. A Medline search was performed for the terms deep-vein thrombosis, postthrombotic syndrome, and thrombolysis for the years 2000–2009.
This review describes the methods, safety, and efficacy of acute interventional treatment of suprapopliteal DVT.
Background
The incidence of clinically recognized acute DVT in the United States is estimated to be up to 250,000 [1] per year, which approaches 1% of the total population.
Thrombi usually develop in the calf veins, propagating in the direction of blood flow subject to the observations of Virchow, who described a triad of blood constituents, blood flow, and vessel wall integrity as the determinants for thrombosis formation. DVTs are classified relative to their involvement of the popliteal vein. DVTs confined below the popliteal vein are rarely associated with adverse outcomes. However, when thrombus extends above the popliteal vein, it may involve the iliac veins or the inferior vena cava with an increased risk of pulmonary embolism and phlegmasia cerulea dolens. More than 90% of symptomatic pulmonary embolism originates from leg veins, and potentially fatal pulmonary embolism will develop in 10% of patients presenting with venous thrombosis above the popliteal vein [1].
The most common risk factors for DVT are recent surgery or hospitalization [2]. However, increasing numbers of patients present with DVT related to other risk factors, including advanced age, increased body mass index, infection, immobilization, use of combined (estrogen-containing) forms of hormone contraception, tobacco use, and long-distance travel associated with a combination of immobility and relative dehydration [3].
The risk of recurrent DVT varies depending on whether the patient has had an idiopathic DVT [4–7], a DVT in the setting of a transient risk factor [8], a permanent risk factor for recurrent DVT, or a history of multiple previous thromboses [9, 10]. Thrombophilia, although uncommon, often expresses itself with recurrent thromboses and should always be excluded by coagulation studies in patients with DVT.
Almost 90% of patients with DVT will have some symptoms, and 15% of these will develop stasis ulcers despite anticoagulation therapy as a result of venous hypertension that results from residual venous obstruction and valve incompetence [11]. This chronic venous insufficiency, most commonly manifesting as postphlebitic syndrome, occurs to some degree in up to 75% of patients with proximal DVT [12] and is more severe in those with iliofemoral DVT compared to infrainguinal DVT. This syndrome is not specifically defined but consists of edema, leg pain, hyperpigmentation, and cutaneous ulceration; it has an incidence of 25%, even when compression stockings are worn for up to 2 years. In those who do not wear compression stockings, however, the incidence of postphlebitic syndrome is up to 60% [13]. The severity of chronic venous disease can be classified according to CEAP, which assesses clinical severity, etiology, anatomy, and pathophysiology with a spectrum ranging from no evidence for venous disease to open venous ulceration. Its use helps promote consistency between physicians and improve accuracy of diagnosis.
There is level III intervention evidence [14–17] that earlier, more complete removal of thrombus will result in reduced DVT recurrence and its subsequent complications, including postphlebitic syndrome, and will improve quality of life [11, 18, 19].
Pathophysiology and History of Treatment
Normal physiology regulates the dissolution of fibrin clots and balances the procoagulant activity of the coagulation proteins. This fibrinolytic system results in a variable degree of recanalization of a thrombosed vein. The active fibrinolytic agent is plasmin, a serine protease that circulates in an inactive form: plasminogen. Plasminogen activators are modulated by several negative regulatory proteins, of which the principal one is α-2-antiplasmin. The degree of recanalization, however, may not be sufficient for complete resolution of clinical symptoms. Sherry described only 6% thrombus lysis with anticoagulation alone [20].
Iliac vein occlusion rarely recanalizes, resulting in persistent iliac obstruction and increased ambulatory venous pressures. This is in contrast to femoropopliteal occlusion by DVT, which can access alternative venous channels. Thus, in the acute setting of suprapopliteal DVT, a threatened limb (phlegmasia) requires aggressive and urgent intervention to avoid limb loss [1]. It is important clinically to recognize that sudden venous outflow obstruction of a limb may cause both “white” and “blue” phlegmasia. Phlegmasia alba dolens, or white, painful thrombosis, is not due to arterial obstruction, as the color suggests, but rather to arterial spasm in response to the marked increase in outflow obstruction caused by the venous thrombosis.
Anticoagulation with heparin from 1937 and warfarin (an oral vitamin K antagonist) from 1954 is still used as a mainstay of treatment for DVT, although more recently, low-molecular-weight heparins have been used in preference to the older preparation.
Patients with suprapopliteal DVT that involves the iliofemoral region continue to be treated in the wider medical community similarly to those with infrainguinal DVT: by anticoagulation therapy without thrombolysis [21]. This is in part because of the bleeding risk of systemic thrombolysis and the apparent nonurgency of clot removal, with adverse sequelae not evident in some patients for up to 15 years [1, 21]. Anticoagulation halts propagation and formation of new thrombus but does not remove existing clots. Early thrombolysis for acute DVT has been shown to deliver improved patient outcomes in terms of symptoms, clot recurrence, and reduced costs [11]. Animal studies have additionally shown that thrombolysis preserves endothelial function and valve competence, and results in more complete thrombus removal [22].
It is recognized that there is an increased bleeding rate in patients on longer-term vitamin K antagonists such as warfarin, with an odds ratio of 2.6 reported in one study [23]. Although the anticoagulant benefit decreases over time, the optimal duration of therapy is unknown, as each patient risk profile is different. In terms of bleeding risk for systemic thrombolysis, early reports suggested an increased number of bleeding episodes [24]; however, more recent higher-quality evidence shows no statistically significant early bleeding risk [25]. Longer-term low-molecular-weight heparin has been compared with oral vitamin K antagonists, showing bleeding rates that are equal or less, and low-molecular-weight heparin is particularly beneficial in patients in whom international normalized ratio control is difficult, or who have malignancy [26].
Surgical thrombectomy with calf massage and intravenous heparin is a method of bulk thrombus removal. However, the indication for operative thrombectomy in the management of acute DVT in the absence of phlegmasia cerulea dolens is unresolved [27]. Although surgical thrombectomy is now rarely performed, in part because of the relatively high recurrence rates compared to catheter-directed thrombolysis, it is more effective than anticoagulation alone [21].
The first clinically useful systemic thrombolytic agent discovered was streptokinase in 1933. Tillett and Garner reported that streptokinase caused complete dissolution of human plasma clot approximately 10 min from contact [28]. Streptokinase activates plasminogen in a two-step process that requires binding to plasminogen to form an activator complex. Apart from the disadvantages of systemic thrombolysis, streptokinase is dependent on an adequate level of plasminogen to bind to, and its effect is reduced as plasminogen is consumed. Because it is a bacterial protein, there is risk of allergy and an incidence of antibody formation, which reduces its effectiveness.
The first agent used to activate plasminogen directly was urokinase, an agent, as its name suggests, that was first extracted from human urine and is now manufactured from mammal-cell cultures [29]. Other direct tissue–plasminogen activators, including alteplase, duteplase, and reteplase, have been developed in an attempt to increase the effectiveness and specificity of thrombolysis.
In the 1990s, as a result of complications relating to systemic thrombolysis, localized treatments emerged. These involve regional clot-directed thrombolysis, ultrasound-enhanced thrombolysis, balloon-isolated thrombolysis, and mechanical clot removal [21, 30].
Interventional Techniques
The more commonly used interventional techniques of thrombus removal include catheter-directed thrombolysis, the pulse-spray technique, and mechanical devices. There are also promising emerging technologies.
Catheter-directed thrombolysis involves thrombolytic infusion through an end-hole catheter in and around the thrombus with or without the operator aspirating the loosened fragments of clot (Fig. 1). The pulse-spray technique was developed in an attempt to increase penetration of the thrombus by lytic agent. This method uses a multiple side-holed catheter that is placed through the thrombus, and lytic drug is intermittently sprayed over several hours until repeat venography is performed and an adequate result achieved.
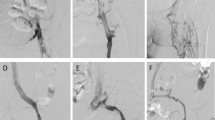
A This patient presented with suprapopliteal deep-vein thrombosis diagnosed clinically and on ultrasound. The femoral vein is nearly completely occluded (open arrow), and there are multiple surrounding venous collaterals (closed arrow) that indicate an established thrombosis. It is unlikely that full clearance with thrombolysis alone will be possible. B Postcatheter-directed thrombolysis and balloon venoplasty. There is ample and rapid flow via the femoral vein (open arrow) with reduced filling of the surrounding collateral veins
Mechanical devices such as the Arrow Trerotola PTD (Arrow, Reading, PA, USA) aim to macerate clot with a spinning metallic helix and aspirate it from the vein. The AngioJet (Possis, Minneapolis, MN) uses burst-sprayed saline with or without thrombolytic to macerate thrombus and create a pressure differential such that clot migrates from vein into catheter. The Trellis system (Bacchus Vascular, Santa Clara, CA) involves isolating thrombus between two inflated balloons, then infusing the area in between the balloons with thrombolytic agent, rotating a sinusoidal wire to fragment the thrombus, which is then aspirated. In patients who have relative contraindications to thrombolysis, these maceration/aspiration systems may be considered, and with the Trellis device, and to a limited extent the Possis device, the thrombolytic agent can be aspirated to prevent systemic release.
Even when interventional radiological techniques are used, long-term anticoagulation remains an essential treatment component to prevent propagation of any persisting clot, help relieve symptoms, and prevent pulmonary embolism [1], although if used alone it yields just 6% complete DVT lysis at 10 days [31]. Systemic thrombolysis results in three times better lysis compared to anticoagulation alone but four times the increased risk of major bleeding [15, 21], prompting a progression to catheter-directed thrombolysis. Despite the technique, a lower success rate is achieved the more chronic the thrombus [21].
There are several developing technologies that are attempting to replace or improve current systems, such as use of plasmin and ultrasound. Although all current thrombolytics are plasminogen activators and dissolve thrombin, plasmin directly degrades fibrin without requiring plasminogen, thus acting locally and reducing bleeding risk. It is also rapidly neutralized, resulting in minimal, if any, systemic effect. Plasminogen activators (current thrombolytics, e.g., t-PA) work proportionally to the amount of plasminogen present in the clot (older clots contain less), but plasmin works regardless of the amount of plasminogen. There is a known threshold effect for bleeding with plasmin, but with plasminogen activators, there is bleeding effect at all doses such that plasmin has a better overall benefit-to-risk profile than plasminogen activators.
Ultrasound thrombus disruption can be used to increase the surface area of fibrin, potentially reducing the dose of thrombolytic required and increasing the rate of lysis (when a thrombolytic such as t-PA is used) [21]. For example, the Ekos EndoWave system (Ekos, Bothell, WA) disaggregates fibrin matrix and exposes additional plasminogen receptor sites to the thrombolytic agent, which may also improve clearance of more chronic thrombus. Furthermore, ultrasound is shown to improve lysis efficiency behind venous valves where thrombus is more difficult to remove.
One study has recently shown clot lysis can also be achieved via microbubbles or nanoparticle drug delivery with, for example, Abciximab (Reopro, Eli Lilly), which is an antiplatelet agent via glycoprotein IIb/IIIa inhibition. Again, a smaller amount of thrombolytic drug can be used and therefore the bleeding risk reduced [32].
Evidence for Interventional Therapy
There is a lack of prospective, randomized data regarding the safety, efficacy, and cost of thrombolytics versus anticoagulants (including the acute procedure, ongoing treatments, and management of complications). Clinical benefit end points in several series are also poorly defined, which makes direct comparison difficult—for example defining significant clot removal or improved patient function. After Comerota and Gravett [21], presented their review data in a tabular form of catheter-directed thrombolysis for acute DVT, we have used a similar approach to compare several more recent studies that use a combination of pharmacomechanical techniques to achieve thrombus resolution (Fig. 2). Notably, in studies covering the last 5 years, there is only a single reported intracranial hemorrhage [33]—previously a significant deterrent for thrombolysis.
Considering the current evidence, the most favorable outcomes are achieved by selecting patients with large suprapopliteal clot burden, phlegmasia, younger age, and May-Thurner syndrome [1]. If a >2-year patient survival is predicted, then immediate thrombus removal should be attempted if appropriate, particularly in active patients with more extensive DVT; this population includes those who are at the highest risk of postthrombotic syndrome [30].
Catheter-directed thrombolysis with local delivery of thrombolytic to the thrombus produces more favorable results than systemic thrombolysis, with almost double the venous patency rate at 1 year and an approximately 80% overall success rate [30]. Aspiration thrombectomy with a catheter and negative syringe pressure is a previously described related technique [12, 34], coupled with inferior vena cava (IVC) filter insertion with or without metallic basket clot fragmentation. Successful clot lysis was seen in nearly 90% of patients but included catheter-directed thrombolysis if the aspiration technique was inadequate or failed. In cases where there is a contraindication to thrombolysis, surgical thrombectomy with or without temporary arteriovenous fistula formation has successfully been performed in order to preserve valve function and reduce the rate of postthrombotic syndrome [17].
As previously mentioned, pharmacomechanical thrombolysis devices including the Trerotola, AngioJet, and Trellis can be used with or without a thrombolytic agent and can involve mechanical maceration or disruption of clot in order to facilitate easier and more complete thrombus aspiration with or without lysis.
Cynamon et al. [35] have reported success with the AngioJet. Fifty percent of patients experienced complete lysis, and 79% of patients had more than half the thrombus cleared (>50% lysis). Complications included 2 out of 24 patients experiencing major hemorrhage, and 1 patient requiring surgery to resolve compartment syndrome (Fig. 3a, b).
A Power-pulse pharmacomechanical thrombolysis with the Poussis device in a patient with acute (3 days) right iliofemoral vein thrombosis. A posterior lumbar fusion operation had been performed several years before, and there was a history of bilateral calf vein deep-vein thrombosis. Initial angiography from the popliteal vein showed complete occlusion with poor collaterals. The thrombus extended up to the confluence of the common iliac veins and was partly outlined with contrast (arrows). The absence of collateral veins indicates an acute thrombosis. B Over a 30-min procedure, the acute thrombus was removed with the Possis AngioJet device to reveal adherent old thrombus and a stenosis related to the lumbar fusion pedicular screws (arrows). C The old thrombus was removed with an Arrow Trerotola PTD device and angioplasty, and 14-mm-diameter nitinol stents were inserted across the stenoses (open arrows). The stents are overlapped to allow for further expansion (middle open arrow). Subsequently, normal flow resulted. There is inflow of unopacified blood in the common femoral vein (closed arrow)
Studies by O’Sullivan et al. [36], Martinez Trabal et al. [37], and Hilleman and Razavi [38] have a combined cohort of 188 patients in whom the Trellis system was used for thrombectomy. Although their complete lysis rates ranged 14–40%, >50% lysis was demonstrated in 92–96%, consistent with a very good and reproducible outcome. In total, there were three major hemorrhage episodes and no procedure-related pulmonary embolism or deaths.
The Clot Buster Amplatz Thrombectomy Device (ATD, Minneapolis, MN) was used by Delomez et al. [39] to achieve a 44% complete lysis rate in patients with iliofemoral DVT. Although there were no major pulmonary embolism or bleeding events reported, all patients experienced transient arterial desaturation such that the procedure was stopped until oxygen saturation recovered. This was thought to be due to micropulmonary emboli, given the inherent nature of the technique to create small, loose thrombi. A single patient presented again with venous ulceration at 6 months. Shi et al. studied the effectiveness of the Amplatz device (and Straub-Rotarex catheter) in a series of 16 patients (18 limbs) with a similar complete lysis rate of 56% [34]. There was a single fatal intracerebral hemorrhage, and one patient with thrombophilia and DVT recurrence developed postthrombotic syndrome.
Although either of these devices may result in pulmonary embolism, evidence from another application, thrombosed dialysis fistula, implies this incidence can be reduced when used in conjunction with a plasminogen activator. A significant incidence of pulmonary embolism has been reported with the pulse-spray technique, evident in 18% of patients who underwent treatment for thrombosed dialysis grafts using plasminogen activator, but increasing to 64% when heparin alone was used. The death rate from pulmonary embolism with catheter-directed thrombolysis, however, does not differ greatly from that seen in DVT cohorts with anticoagulation alone [13, 30].
Percutaneous mechanical thrombectomy and thrombolysis have the benefit of not requiring intensive monitoring of a continuous infusion of thrombolytic agent [37]. However, the risks of vein wall and valve injury may be higher given the mechanical forces exerted by some of the equipment. Pharmacomechanical thrombolysis of a specific region or isolated segment of clot is often quicker and uses reduced thrombolytic dose with much of what is used being aspirated back, reducing the systemically absorbed dose even further (Fig. 3). Compared to catheter-directed thrombolysis alone in the short term, there is a similar incidence of adjunctive venoplasty and stenting, complications, and length of hospital stay. Martinez Trabal et al. [37] and O’Sullivan et al. [36] report similar figures for percutaneous mechanical thrombectomy with the Trellis system, whereby complete clot removal was seen in just 14 and 17% of patients, respectively. However, an improved result is evident in combination with catheter-directed thrombolysis of 32%.
Other potential issues with mechanical thrombolysis devices include a temporary anemia and deterioration in renal function. This is thought to be due to a varying degree of hemolysis, seen, for example, with the Trellis system [21]. O’Sullivan et al. [36] demonstrated a shorter infusion time and reduced dose, and therefore cost, of lytic drug. There were no bleeding episodes reported by Hilleman and Razavi [38], compared to an 8% incidence of major bleeding with catheter-directed thrombolysis. Chronically occluded vessels yielded inferior results [40]. The AngioJet device also causes hemolysis, resulting in decreased white blood cell and platelet counts in porcine models. Lang et al. [40] recommend caution in patients who have recently had a blood transfusion and in children with a lower blood volume and presumably less reserve.
Postthrombolysis
Despite the method of thrombolysis, if an underlying cause or lesion can be found and if the DVT is chronic, angioplasty with or without stenting can help prevent or prolong the interval to recurrence [11, 20]. Stenting an underlying lesion can result in almost 50% improved patency compared with systemic thrombolysis alone [11], with most patients being women, with a female-to-male ratio of 2.6–1 (Fig. 4). Within the iliac veins, 1% of stents can be expected to reocclude within 30 days [41] and a >50% restenosis will be seen in up to 15% of patients at 42 months [11]. Restenosis is more commonly seen in patients who are postthrombotic, have had a previous DVT, are hypercoagulable, or have longer length stents, including those that extend below the inguinal ligament. Overall, however, 92% primary assisted patency at 3 years can be achieved. The issue of stenting below the inguinal ligament in cases of proximal DVT has again been brought to light [42], with >50% stenosis seen in just 5% of all stents at 32 months.
A Left external iliac vein stenosis with a small amount of thrombus (arrows). Note the multiple inguinal collateral venous pathways, indicating a significant gradient. B Status after single end-hole catheter-directed thrombolysis and a long nitinol stent placement (arrows). Because the venous gradient has disappeared, the collaterals are no longer filled with contrast
Other Considerations
May-Thurner Syndrome
Iliac vein compression syndrome is typically left-sided with a compressed proximal left common iliac vein between the right common iliac artery anteriorly and vertebral bodies posteriorly (Fig. 5). Vein fibrosis develops over time as a result of the pulsing artery-on-vein mechanical trauma, endothelial irritation, and subsequent formation of venous spurs and synechiae. It usually becomes evident in women during their third or fourth decades. Magnetic resonance Venogram/computed tomographic angiogram can help in the diagnosis, where one may see a >70% compression of the left common iliac vein. In May-Thurner syndrome, when the underlying obstruction is not treated with a stent, there is a reported 73% recurrence of acute left iliofemoral DVT [43].
A Recurrent deep-vein thrombosis due to May-Thurner syndrome diagnosed by ultrasound in a 23-year-old woman. Stenosis confirmed with venography showed the typical indentation caused by the right common iliac artery as it crosses the left common iliac vein (arrows). B Balloon venoplasty was performed (arrows) and resulted in continued patency with 3-year Doppler ultrasound follow-up. Because 27% will respond to balloon venoplasty alone, in a young patient, avoidance of a stent should be considered
Children
There is less evidence available about the investigation and treatment of suprapopliteal DVT in children. A review article by Brightwell and Osman [44] summarizes the available evidence, most appropriate investigations, and treatment options. They report a bimodal distribution for thromboembolic disease in children that is seen in the neonatal period; it is thought to be due to a lack of fibrinolytic factors, with a second peak at puberty due to reduced fibrinolytic activity, resulting in an overall pediatric incidence of approximately 0.5%. Relatively high incidence is seen within the first year of life in hospitalized patients as a result of central venous access devices. The patient most commonly experiences pain and swelling in the affected limb.
Ultrasound is considered the most appropriate first-line investigation for pediatric thrombus; however, venography remains the gold standard, with magnetic resonance imaging or computed tomography encouraged as adjuncts, thanks to better definition of the surrounding anatomy. The child should be assessed for prothrombotic risk factors, including blood screening. Although there is a lack of evidence for treatment options in children, at this stage, a similar algorithm to that used in adults can be used with initial therapy consisting of immediate heparin and a long-term vitamin K antagonist such as warfarin. There are no study data to support thrombolysis, whether pharmacological or mechanical, although it and surgical thrombectomy can be considered in patients with high thrombus load where there is threatened loss of life or limb. Prophylactic optional/removable IVC filters are also being used in select patients, such as those with contraindications to anticoagulation or with progressive DVT/pulmonary embolism despite anticoagulation.
Pregnancy
Venous thromboembolism affects up to 3 per 1000 pregnant women annually—a rate 10 times more common than in nonpregnant women [45, 46]. This can be attributable to hemostatic changes as well as both inherited and acquired maternal thrombophilias. Treatment options must be carefully considered, given the possible inherent hemorrhagic and teratogenic repercussions for both mother and baby.
Therapy is preferably with low-molecular-weight heparin [47]. Warfarin should not be used, especially between 6 and 12 weeks’ gestation, and after 36 weeks. Therapy should continue to 6 weeks postpartum, for an overall minimum term of 6 months [48]. There is a lack of data regarding thrombolysis, catheter-directed thrombolysis, and surgical embolectomy in pregnant patients, with several reviews and case reports only [49]. If systemic thrombolysis is to be used, Doreen te Raa et al. [49] have recently recommended rt-PA rather than streptokinase or urokinase because of fewer bleeding complications, shorter duration of administration, and no allergic complications.
Surgical embolectomy should be considered when there are contraindications to conventional or thrombolytic therapy, but it should be limited to centers with adequate surgical and perioperative support. Although it is a rarely performed procedure, maternal mortality seems low, although there is a higher rate of fetal death and preterm delivery. Even more rarely reported is catheter-directed thrombolysis/thrombectomy in pregnancy. One fetal death and one preterm delivery resulted in four cases reviewed by Doreen te Raa et al. [49]. Further evidence is clearly required before a consensus recommendation can be considered.
Recommendations for Treatment
In patients with recent internal bleeding, severe hypertension, or recent cardiovascular accident, thrombolysis of suprapopliteal DVT is contraindicated and should only be undertaken after careful consideration of the overall benefit-to-risk profile [11].
With catheter-directed thrombolysis, major bleeding complications have been reported in 11% of patients, 39% of these at the puncture site, yet only 0.4% intracranially. An overall mortality of 0.4% is predominantly from pulmonary embolism and intracranial hemorrhage [11]. Despite a 10% incidence of pulmonary embolism, only 1% has been demonstrated to be symptomatic, suggesting routine IVC filter insertion may be unwarranted and necessary only in particular cases such as reduced cardiopulmonary reserve or free-floating thrombus.
Currently, patients with suprapopliteal DVT should undergo immediate anticoagulation and thrombus removal if appropriate; they should wear long leg compression stockings [9, 18] and elevate the leg when not ambulant. Computed tomographic scans of the chest, abdomen, and pelvis can help exclude pulmonary embolism and causative pathologies such as malignancy. If there is IVC thrombus or free-floating clot on ultrasound, an IVC filter may be inserted (Fig. 6). Similarly, insertion can be performed if there is new major thrombus despite adequate anticoagulation [1]. Unfortunately, most patients with iliofemoral thrombosis are still managed on a standard anticoagulation protocol, with no immediate thought of thrombus removal (Fig. 7). We propose an alternative pathway, with priority given to reduction of thrombus burden. The exact technique of thrombolysis will vary according to local conditions and availability of equipment. Appendix 1 provides our guidelines as an example.
A Typical patient presentation with extensive iliofemoral deep-vein thrombosis (DVT) after standard medical therapy for DVT. An optional Cook Celect IVC filter was inserted before catheter-directed thrombolysis. The tip-occluding wire of the thrombolysis catheter is positioned adjacent to the tip of the IVC filter (open arrow). Thrombus (closed arrow) extended up to the filter. The central metallic artefact overlying the thrombus is due to umbilical jewelry. B Status after catheter-directed thrombolysis in the same patient after removal of the pulse-spray catheter. There is minor residual thrombus in the inferior portion of the inferior vena cava and proximal left common iliac vein (closed arrows)
Thrombus should be treated acutely. Although treatment of arterial thrombosis is aimed at a 2–6-h window from onset in stroke and myocardial ischemia [50], the tissue at risk with venous thrombosis is far more tolerant to ischemia, and a time period of days rather than hours is acceptable. There are no data to support use of thrombolytic drugs in localized infrapopliteal venous thrombosis, although a single-dose technique is used for cardiac and carotid artery thrombosis with positive outcomes. Current thinking is that the drug would be directed away from the area of venous thrombosis by blood flow. This hypothesis remains to be tested.
If no underlying lesion can be found, then no further intervention is necessary, although thrombophilia screening is essential. If there is an underlying iliac lesion, then venoplasty can be performed with or without stenting if required to maintain patency; however, if a femoral lesion is found, venoplasty alone is preferable. Some centers prescribe aspirin and/or clopidogrel for up to 6 weeks after insertion of an iliac stent [1], although the data supporting the use of clopidogrel specifically relate to reduced intimal hyperplasia in coronary stents [51].
After acute anticoagulation with heparin and then thrombolysis, long-term heparin, or warfarin (international normalized ratio 2–3) is recommended. Duration should be for at least 3 months in the setting of transient risk factors, but at least 12 months if there has been DVT recurrence [10]. Treatment up to 2 years has been shown to be of benefit [18], with fewer recurrences the longer the treatment [5, 9, 52, 53], although there is an increased number of major bleeding events. Such effective thrombus resolution for patients on long-term anticoagulation resulted in a trial being ceased because it was no longer ethical to prescribe placebo instead of anticoagulant [4, 10]. When determining optimal treatment duration, the overall individual risk-to-benefit profile must be considered.
Conclusion
Iliofemoral DVT can be safely and effectively treated at presentation in most patients with a combination of catheter-directed thrombolysis and/or mechanical thrombectomy devices. There is mounting evidence that this approach preserves venous valve function, reduces longer-term venous hypertension, and lowers the incidence of postthrombotic syndrome. After the acute episode, anticoagulation is required, with duration dependent on many patient factors, including the presence of an underlying pathology. None of the evidence available at this time is of a high enough level to develop clear guidelines for a specific treatment pathway. Prospective, randomized trials are required, and to this end, the NHLBI (USA) has funded the ATTRACT (Acute Venous Thrombosis: Thrombus Removal With Adjunctive Catheter-directed Thrombolysis) trial, which commenced enrollment in late 2009 at 35 U.S. centers.
Solitary minor, or below-popliteal, DVT can be safely treated with compression stockings and anticoagulation without the need for urgent intervention such as catheter-directed thrombolysis or mechanical thrombectomy, given the associated low morbidity and mortality.
New thrombolytics and delivery devices continue to improve clot lysis and removal; however, the patient risk-to-benefit profile must always be considered before any technique is routinely used in practice.
References
Butani D, Waldman DL (2008) Catheter directed thrombolysis for iliofemoral deep vein thrombosis. Oper Tech Gen Surg 10:143–148
Qaseem A, Snow V, Barry P et al (2007) Current diagnosis of venous thromboembolism in primary care: a clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians. Ann Fam Med 5:57–62
Segal JB, Eng J, Jenckes MW et al (2003) Diagnosis and treatment of deep venous thrombosis and pulmonary embolism. Evid Rep Technol Assess (summer):1–6
Decousus H, Leizorovicz A, Parent F et al (1998) A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prévention du Risque d’Embolie Pulmonaire par Interruption Cave Study Group. N Engl J Med 338:409–415
White RH, Zhou H, Kim J et al (2000) A population-based study of the effectiveness of inferior vena cava filter use among patients with venous thromboembolism. Arch Intern Med 160:2033–2041
Levine MN, Hirsh J, Gent M et al (1995) Optimal duration of oral anti-coagulant therapy: a randomized trial comparing four weeks with three months of warfarin in patients with proximal deep vein thrombosis. Thromb Haemost 74:606–611
Schulman S, Granqvist S, Holmström M et al (1997) The duration of oral anticoagulant therapy after a second episode of venous thrombo-embolism. The Duration of Anticoagulation Trial Study Group. N Engl J Med 336:393–398
Kearon C, Gent M, Hirsh J et al (1999) A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med 340:901–907
Prandoni P, Lensing AW, Prins MH et al (2004) Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med 141:249–256
Snow V, Qaseem A, Barry P et al (2007) Management of venous thromboembolism: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Fam Med 5:74–80
Hood DB, Alexander JQ (2004) Endovascular management of iliofemoral venous occlusive disease. Surg Clin N Am 84:1381–1396
Kwon SH, Oh JH, Seo TS et al (2009) Percutaneous aspiration thrombectomy for the treatment of acute lower extremity deep vein thrombosis: is thrombolysis needed? Clin Rad 64:484–490
Vedantham S, Millward SF, Cardella JF et al (2006) Society of Interventional Radiology position statement: treatment of acute iliofemoral deep vein thrombosis with use of adjunctive catheter-directed intrathrombus thrombolysis. J Vasc Interv Radiol 17:613–616
National Health and Medical Research Council of Australia (2009) NHMRC additional levels of evidence and grades for recommendations for developers of guidelines. Stage 2 pilot. Available at: http://www.nhmrc.gov.au/guidelines/consult/consultations/add_levels_grades_dev_guidelines2.htm. Accessed April 25, 2010
Goldhaber SZ, Buring JE, Lipnick RJ, Hennekens CH (1984) Pooled analyses of randomized trials of streptokinase and heparin in phlebographically documented acute deep venous thrombosis. Am J Med 76:393–397
Plate G, Akesson H, Einarsson E et al (1990) Long-term results of venous thrombectomy combined with a temporary arterio-venous fistula. Eur J Vasc Surg 4:483–489
Plate G, Einarsson E, Ohlin P et al (1984) Thrombectomy with temporary arteriovenous fistula: the treatment of choice in acute iliofemoral venous thrombosis. J Vasc Surg 1:867–876
Merli GJ (2008) Treatment of venous thromboembolism. Am J Med 121(11A):S2–S9
AbuRahma AF, Perkins SE, Wulu JT (2001) Iliofemoral deep vein thrombosis: conventional therapy versus lysis and percutaneous transluminal angioplasty and stenting. Ann Surg 233:752–760
Sherry S (1985) Thrombolytic therapy for deep venous thrombosis. Semin Intervent Radiol 4:331–337
Comerota AJ, Gravett MH (2007) Iliofemoral venous thrombosis. J Vasc Surg 46:1065–1076
Rhodes JM, Cho JS, Gloviczki P (2000) Thrombolysis for experimental deep venous thrombosis maintains valvular competence and vasoreactivity. J Vasc Surg 31:205–1193
Hutten BA, Prins MH (2006) Duration of treatment with vitamin K antagonists in symptomatic venous thromboembolism. Cochrane Database Syst Rev (1):CD001367
Ng CM, Rivera JO (1998) Meta-analysis of streptokinase and heparin in deep vein thrombosis. Am J Health Syst Pharm 55:1995–2001
Watson L, Armon MP (2004) Thrombolysis for acute deep vein thrombosis. Cochrane Database Syst Rev (3):CD002783
Segal JB, Streiff MB, Hofmann LV et al (2007) Management of venous thromboembolism: a systematic review for a practice guideline. Ann Intern Med 146:211–222
Greenfield LJ, Alexander EL (1985) Current status of surgical therapy for deep vein thrombosis. Am J Surg 150(4A):64–70
Tillett WS, Garner RL (1933) The fibrinolytic activity of haemolytic streptococci. J Exp Med 58:485–502
Roychoudhury PK, Khaparde SS, Mattiasson B, Kumar A (2006) Synthesis, regulation and production of urokinase using mammalian cell culture: a comprehensive review. Biotechnol Adv 24:514–528
Comerota AJ, Paolini D (2007) Treatment of acute iliofemoral deep venous thrombosis: a strategy of thrombus removal. Eur J Vasc Endovasc Surg 33:351–360
Alonso A, Dempfle C, Martina A et al (2009) In vivo clot lysis of human thrombus with intravenous abciximab immunobubbles and ultrasound. Thromb Res 124:70–74
Kim BJ, Chung HH, Lee SH (2010) Single-session endovascular treatment for symptomatic lower extremity deep vein thrombosis: a feasibility study. Acta Radiol 51:248–255
Chang R, Chen CC, Kam A et al (2008) Deep vein thrombosis of lower extremity: direct intraclot injection of alteplase once daily with systemic anticoagulation—results of pilot study. Radiology 246:619–629
Shi HJ, Huang YH, Shen T et al (2009) Percutaneous mechanical thrombectomy combined with catheter-directed thrombolysis in the treatment of symptomatic lower extremity deep venous thrombosis. Eur J Radiol 71:350–355
Cynamon J, Stein EG, Dym RJ, Jagust MB, Binkert CA, Baum RA (2006) A new method for aggressive management of deep vein thrombosis: retrospective study of the power pulse technique. J Vasc Interv Radiol 17:1043–1049.
O’Sullivan GJ, Lohan DG, Gough N et al (2007) Pharmacomechanical thrombectomy of acute deep vein thrombosis with the trellis-8 isolated thrombolysis catheter. J Vasc Interv Radiol 18:715–724
Martinez Trabal J, Comerota A, LaPorte F et al (2008) The quantitative benefit of isolated, segmental, pharmacomechanical thrombolysis (ISPMT) for iliofemoral venous thrombosis. J Vasc Surg 48:1532–1537
Hilleman DE, Razavi MK (2008) Clinical and economic evaluation of the Trellis-8 infusion catheter for deep vein thrombosis. J Vasc Interv Radiol 19:377–383
Delomez M, Beregi JP, Willoteaux S, Bauchart JJ, Janne d’Othée B, Asseman P, Perez N, Théry C (2001) Mechanical thrombectomy in patients with deep venous thrombosis. Cardiovasc Intervent Radiol 24:42–48
Lang EV, Kulis AM, Villani M et al (2008) Hemolysis comparison between the OmniSonics OmniWave Endovascular System and the Possis AngioJet in a porcine model. J Vasc Interv Radiol 19:1215–1221
Neglén P, Raju S (2004) In-stent recurrent stenosis in stents placed in the lower extremity venous outflow tract. J Vasc Surg 39:181–188
Neglén P, Tackett TP Jr, Raju S (2008) Venous stenting across the inguinal ligament. J Vasc Surg 48:1255–1261
Oguzkurt L, Ozkan U, Ulusan S et al (2008) Compression of the left common iliac vein in asymptomatic subjects and patients with left iliofemoral deep vein thrombosis. J Vasc Interv Radiol 19:366–371
Brightwell RE, Osman IS (2006) Iliofemoral deep vein thrombosis in childhood: developing a management protocol. Eur J Vasc Endovasc Surg 31:667–677
Lindqvist P, Dahlback B, Marsal K (1999) Thrombotic risk during pregnancy: a population study. Obstet Gynecol 94:595–599
Toglia MR, Weg JG (1996) Venous thromboembolism during pregnancy. N Engl J Med 335:108–114
Greer IA, Nelson-Piercy C (2005) Low-molecular-weight heparins for thromboprophylaxis and treatment of venous thromboembolism in pregnancy: a systematic review of safety and efficacy. Blood 106:401–407
Clark P, Bates SM (2009) North American and British guidelines for anti-thrombotic therapy: are we reaching consensus? Thromb Res 123:S111–S123
Doreen te Raa G, Ribbert L, Snijder R et al (2009) Treatment options in massive pulmonary embolism during pregnancy: a case-report and review of literature. Thromb Res 124:1–5
Tsivgoulis G, Culp WC, Alexandrov AV (2008) Ultrasound enhanced thrombolysis in acute arterial ischemia. Ultrasonics 48:303–311
Gurbel PA, Bliden KP, Hiatt BL, O’Connor CM (2003) Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation 107:2908–2913
Ginsberg JS, Hirsh J, Julian J et al (2001) Prevention and treatment of postphlebitic syndrome: results of a 3-part study. Arch Intern Med 161:2105–2109
Schulman S, Rhedin AS, Lindmarker P et al (1995) A comparison of six weeks with six months of oral anticoagulant therapy after a first episode of venous thromboembolism. Duration of Anticoagulation Trial Study Group. N Engl J Med 332:1661–1665
Hann CL, Streiff MB (2005) The role of vena caval filters in the management of venous thromboembolism. Blood Rev 19:179–202
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1
Notes on iliofemoral deep-vein thrombosis thrombolysis technique
Choice of Access
Because it is uncommon to find a patent femoral vein on the same side as an iliac vein thrombosis, in most cases, the easiest point of access is with the patient prone, from the popliteal or sural veins. These veins can be identified with ultrasound, and because the passage of a guide wire is in the normal direction of flow, the venous valves present no impediment.
In other cases where a contralateral femoral approach is used, it can be difficult to advance a catheter into the deep femoral veins and to negotiate the venous valves, which occur at approximately 3–4-cm intervals. Access from the right internal jugular vein is also possible, but longer catheters are required, and in the femoral segment, the same issue of venous valve passage applies.
If an inferior vena cava filter is to be placed before thrombolysis, the use of the internal jugular route allows the least possible interference with any thrombus in the inferior vena cava. A retrievable filter with an extended retrieval time should be used.
Puncture of the popliteal vein under ultrasound avoids the risk of arterial puncture. In most cases, the artery lies posterior and medial to the popliteal vein. Placement of a 7F sheath allows injection of a small amount of contrast to determine the inferior extent of the thrombus.
Our preferred technique is to advance a Terumo Glide guide wire up the thrombosed segment, followed by a 5F catheter with a “hockey stick” or “cobra2” tip configuration. This allows the guide wire–catheter combination to be steered into the desired position. The uppermost level of the thrombus is defined by passage of the catheter into the inferior vena cava unless there is thrombus in the inferior vena cava (IVC) already.
At this stage, the choice of how to treat the thrombus with lytic agent (t-PA or urokinase) is the following:
-
Inject aliquots of lytic agent with the original catheter as it is withdrawn. (This technique is less suitable for large thrombus volumes because larger volumes of lytic agent are required.)
-
Replace the original catheter with a pulse-spray catheter of a treatment length that is at least as long as the thrombosed segment (maximum is 60 cm).
-
Possis device in “power-pulse” and wait mode. This mode does not aspirate the thrombus; it simply provides agitation and mixing of thrombus and lytic agent.
-
Trellis device agitated lysis, wait, and aspirate.
Which of these is used is a personal preference based on cost and availability. (We use the Possis device because the lysed thrombus can be aspirated within 30 min and longer infusions are not required, but the operation of the Trellis device is very similar.)
The lytic agent needs time to activate plasmin and for thrombolysis to occur. With pulse-spray catheters and large thrombus loads, this is most conveniently left overnight and the patient returned the next day for completion.
With overnight pulse-spray infusions, relatively large doses of lytic agent (1–2 million units of urokinase and 10,000 IU heparin) are required, and even so, it is not uncommon to find that significant residual thrombus remains. Often a mechanical device or angioplasty is required to establish patency. This has led to greater use of mechanical devices such as the Poussis and Trellis devices.
The Possis device and Trellis device are capable of rapid lysis because of the more effective agitation of thrombus and the ability to aspirate the thrombus once it is lysed. Typically we would use 200–500,000 U of urokinase and 5000 IU heparin in the Possis device, depending on the estimated thrombus burden.
If there is residual old thrombus (older than 7–10 days), an Arrow Trerotola PTD device or balloon angioplasty may be used to clear the lumen, and if necessary, any luminal stenosis may be stented with a 12–16-mm diameter self-expanding nitinol stent.
These mechanical devices allow the thrombolytic procedure to be completed in 30–90 min. The attraction of the single procedure is that hospital stay is minimized and complications of bleeding are less likely. The 7F sheath is removed at the end of the procedure and the affected limb elevated 30 degrees.
Aftercare
An overnight admission is usual, and the affected leg should have returned to a more normal appearance by morning. A degree of hemolysis is expected; it is related directly to the volume of thrombus treated by mechanical devices.
After flow is established angiographically and there is no significant residual obstruction from stenosis or thrombus, low-molecular-weight heparin and warfarin therapy are begun. Oral anticoagulation (international normalized ratio 2.5–3.0) is continued for 3–12 months. The duration of anticoagulation depends largely on the response, patient compliance, and the managing hematologist’s opinion. Once the affected leg is of normal caliber, compression stockings are fitted. On the basis of the experience of Prandoni et al. [9], patients are encouraged to wear these stockings for a lengthy period, and at least until the period of oral anticoagulation is completed.
Review with ultrasound is only indicated in the event of a return of symptoms. Patients who have had a DVT are very aware of how their legs should feel.
IVC Filters
Whether to use a protective IVC filter is still controversial, and no high-level data exist [54]. As part of the informed consent process, one should offer the patient the choice of filter or not, as the risk of filter insertion and retrieval is low.
Most people who have performed thrombolysis with IVC thrombus have seen evidence of pulmonary embolism. Although theoretically the embolus will have some lytic agent within it, this is little comfort if a large embolus occurs.
The risk in our experience seems to be higher with prolonged pulse-spray lysis and when there is significant thrombus within the IVC itself.
In the case of a localized iliac or iliofemoral thrombus with normal lung function, we would not normally insert a filter. If an IVC filter is used, it should be one that can be easily retrieved once the thrombotic episode has been resolved.
Rights and permissions
About this article
Cite this article
Pianta, M.J., Thomson, K.R. Catheter-Directed Thrombolysis of Lower Limb Thrombosis. Cardiovasc Intervent Radiol 34, 25–36 (2011). https://doi.org/10.1007/s00270-010-9877-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-010-9877-z