Abstract
Introduction
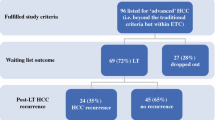
The drop-off risk for patients awaiting liver transplantation for hepatocellular carcinoma (HCC) is 22%. Transplant liver availability is expected to worsen, resulting in longer waiting times and increased drop-off rates. Our aim was to determine whether chemoembolization can decrease this risk.
Patients and Methods
Eighty-seven consecutive HCC patients listed for liver transplant (Milan criteria) underwent statistical comparability adjustments using the propensity score (Wilcoxon, Fisher’s, and chi-square tests). Forty-three nonchemoembolization patients and 22 chemoembolization patients were comparable for Child-Pugh and Model for End-Stage Liver Disease scores, tumor size and number, alpha fetoprotein (AFP) levels, and cause of cirrhosis. We calculated the risk of dropping off the transplant list by assigning a transplant time to those who dropped off (equal probability with patients who were on the list longer than the patient in question). The significance level was obtained by calculating the simulation distribution of the difference compared with the permutations of chemoembolization versus nonchemoembolization assignment of the patients. Kaplan–Meier estimators (log-rank test) were used to determine survival rates.
Results
Median follow-up was 187 ± 110 weeks (range 38 to 435, date of diagnosis). The chemoembolization group had an 80% drop-off risk decrease (15% nonchemoembolization versus 3% chemoembolization, p = 0.04). Although survival was better for the chemoembolization group, it did not reach statistical significance. Two-year survival for the nonchemoembolization and chemoembolization group was 57.3% ± 7.1% and 76.0% ± 7.9%, respectively (p = 0.078).
Conclusions
Chemoembolization appears to result in a significant decrease in the risk of dropping off liver transplant list for patients with HCC and results in a tendency toward longer survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The implementation of the Milan [1] and San Francisco [2] (orthotopic) liver transplantation (OLT) criteria for patients with hepatocellular carcinoma (HCC) has resulted in significantly improved postsurgical survival rates [3]. Currently, the post-OLT 5-year survival rate is approximately 70% to 80% [2, 5–7], with a disease-recurrence rate of 15% [3–5]. Because of these excellent long-term results, the percent of OLTs performed for cirrhosis complicated by HCC has increased from 7% to 22% and is bound to increase further as the incidence of HCC continues to climb [5, 7–11]. Another breakthrough occurred with the introduction of the Model for End-Stage Liver Disease (MELD) allocation system in 2002, which decreased the mean liver transplant wait list period from 27.7 months to 8.3 months [7]. Despite this decrease, the drop-off rate among patients awaiting liver transplantation continues to be unacceptably high at approximately 22% overall and 11%, 57%, and 75% at 6, 12, and 18 months respectively [7]. Recent data suggest that the strict Milan criteria can be expanded to patients with (1) a single tumor <6.5 cm or (2) 3 tumors, each <4.5 cm and with a total diameter <8 cm (San Francisco criteria) without significantly compromising survival rates [6, 8]. Although this will allow more patients to potentially benefit from the only treatment option that cures both the HCC and the underlying cirrhosis, it is certain to further increase the wait-list period and consequently the drop-off rate. Despite attempts to increase the donor pool, liver transplant waiting lists are growing longer both in Europe and the United States [10, 12], putting a heavier burden on pre-OLT interventions. Chemoembolization has been shown to provide a survival benefit to patients with unresectable HCC [13–15] and to result in significant tumor responses according to both European Association for the Study of the Liver (EASL) and Response Evaluation Criteria In Solid Tumors (RECIST), [8, 10, 16]. It has occasionally been employed as a bridging treatment for patients on the liver transplantation waiting list, hoping either to decrease the drop-off risk or to improve post-OLT survival, but has had mixed results [4, 5, 8, 10, 11, 16–23]. Our objective was to establish whether treating patients who are on the liver transplantation waiting list for HCC with chemoembolization decreases their drop-off risk.
Patients and Methods
Patients
The treatment received by all patients was part of standard clinical care, and relevant informed consent was obtained. This was a retrospective study that conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the appropriate Institutional Review Committee. From 2001 to 2008, a total of 87 patients (61 male and 26 female; median age of 53 ± 8 years [range 33 to 72]) were listed for liver transplantation because of cirrhosis-related HCC. All patients fulfilled the Milan criteria (single lesion <5 cm diameter or ≤3 lesions <3 cm in diameter). Diagnosis of HCC was made based by biopsy specimen or the presence of a hypervascular liver lesion in a cirrhotic liver and an alpha fetoprotein (AFP) >400 ng/ml. Data analysis was performed in late 2009, and by then all patients either undergone OLT or had dropped off the liver transplant list. For all patients, we recorded the cause of cirrhosis, Child-Pugh (C-P) and MELD scores, tumor morphology on dual-phase computed tomography (CT) or magnetic resonance imaging (MRI), AFP scores, and, if appropriate, the reason for drop off and cause of death. Of the 87 patients listed for liver transplantation for cirrhosis-related HCC, 52 did not undergo chemoembolization while on the waiting list, and 35 were treated according to the Johns Hopkins chemoembolization protocol (see later text). All patients were presented at our weekly multidisciplinary Liver Tumor Board, where interventional radiology, liver surgery, hepatology and oncology disciplines are represented, and the plan for liver transplant listing and chemoembolization or nonchemoembolization was formulated. Inclusion criteria were as follows: (1) lesion size >2 cm, (2) a lesion ≤1 cm from the planned resection margin, and (3) a lesion ≤1 cm from a major blood vessel that needed to be preserved. All chemoembolizations were performed by experienced interventional radiologists.
Chemoembolization Protocol
Patients undergoing chemoembolization had a baseline dual-phase liver MRI. Chemoembolization was performed with a 5F catheter placed in the right or left hepatic artery. Whenever the tumor vascular supply allowed, a more selective chemoembolization was performed with the catheter tip in a segmental hepatic arterial branch. A 7- to 10-mL chemotherapy solution was infused―cisplatin 100 mg (Bristol Myers Squibb, Princeton, NJ), doxorubicin 50 mg (Adriamycin; Pharmacia-Upjohn, Kalamazoo, MI), and mitomycin C 10 mg (Bedford Laboratories, Bedford, OH)―in a 1:1 volume ratio with Ethiodol (Savage Laboratories, Melville, NY) or in a 2:1 ratio with twice as much chemotherapy as Ethiodol, depending on flow characteristics, to avoid complete stasis within the selected hepatic artery. This was followed by infusion of 1 to 4 mL Embosphere particles (Biosphere Medical, Boston, MA) measuring 100 to 300 μm in size to slow arterial inflow and prevent wash of the chemotherapeutic agents. The end point of the procedure (technical success) was achieved when the entire amount of chemotherapy was delivered and the infused Embospheres resulted in visibly slowed arterial flow. Nonbuffered lidocaine (10 to 20 cc, 1:100 ratio) was also given intra-arterially after chemoembolization for pain control. After chemoembolization, patients were admitted overnight, and a non-contrast-enhanced CT scan of the liver was obtained to document Ethiodol distribution before the patient was discharged. Technical success was defined as completion of the chemoembolization procedure and the follow-up nonenhanced CT showing distribution of Lipiodol in the targeted lobe or segment. The treatment protocol (dual-phase liver MRI and chemoembolization) was repeated every 4 to 6 weeks. If MRI showed >90% tumor necrosis, chemoembolization was postponed until re-evaluation with follow-up MRI showed <90% tumor necrosis. Exclusion criteria for chemoembolization were total bilirubin >4 mg/dl, C-P score of C, or Eastern Collaborative Oncology Group performance status of 3.
Statistical Analysis
Our primary objective was to estimate the risk of dropping off the transplant list for those patients treated with chemoembolization, compare it with the risk of those patients not treated with chemoembolization, and to determine whether any difference was statistically significant. The drop-off risk was calculated prospectively at time of listing for all patients expected to wait for a transplant equal or longer than m months (m = 1, 2,…, 28). To calculate the drop-off risk, the following two issues had to be addressed: First, we had to ensure that the two groups (chemoembolization vs. no chemoembolization) were matched. Second, to determine the risk, we had to calculate the expected time of transplant for those patients who dropped off the list had they not dropped off.
Matching
To address the possibility that the two groups were not comparable, we first compared them using Wilcoxon test for ordinal variables, Fisher’s exact test for nominal binary variables, and chi-square test for nominal variables with more than two levels. Because the groups differed in C-P score, size of tumor, and AFP level (Table 1), we estimated the propensity score [24] of being in the chemoembolization versus the nonchemoembolization group as function of these variables. Nine of the 52 patients in the nonchemoembolization group and 13 of the 35 patients in the chemoembolization group had no propensity-comparable patient in the contralateral group and were removed from further analysis. The remaining 22 chemoembolization and 43 nonchemoembolization patients were thus matched for all variables in Table 1 were and were used in the subsequent analysis to calculate the drop-off risk.
Drop-Off Risk Calculation
To calculate drop-off risk, first the expected transplantation time for patients that dropped off the transplant list must be estimated. The expected waiting time for a patient who dropped off the list, was assigned with equal probability as the actual waiting time for patients who were on the list at least as long as the patient in question. We then calculated the risk of dropping off, as defined above, for the chemoembolization and nonchemoembolization groups. The difference between the drop-off risk between the groups, as well as the significance level for this difference, was obtained by calculating the simulation distribution of the difference compared with the permutations of chemoembolization versus nonchemoembolization assignment of the patients. For a detailed explanation of this method, the reader is referred to Rosenbaum [25]. We also calculated the risk of dropping off the transplant list using Kaplan–Meier estimators. This method compares the two groups overall. In contrast, the above-described propensity score method calculates the drop-off risk in relation to time on the waiting list. This is important because the drop-off risk correlates strongly with time spent on the waiting list. Survival rates were calculated using Kaplan–Meier estimators, and patients alive at time of analysis were censored; the Kaplan–Meier curves were compared between treated and untreated patients using log-rank test.
Sample Size Calculation
For the statistical method used, sample size or power cannot be accurately calculated before determining the variance of the estimators for the drop-off risks for the two groups. This variance and the sample size and power can be calculated as follows:
Define d as the difference between logit(true drop-off risk in the non-TACE group) and logit(true drop-off risk in the TACE group). Then define D as the estimator of d and assume it is approximately normally distributed as described by Pr(D | d) = Normal { d, v(n) }. Here, v(n) is the variance of D and is inversely proportional to the sample size as is the case in most regular estimators of the asymptotic theory (Here logit(.) is the function log(./(1−.)), which is the inverse of the logistic function).
Then it follows that v(n) = 1/info(n), where info(n) = n*info(1), and where info(1) is the Fisher information number for estimating d based on a single observation (of course, d is inestimable from a single observation; info(1) is merely a rate of change of the Fisher information with sample size). To conduct a sample size calculation, we must first know info(1).
By using the simulation distribution of the estimator D one can calculate p. Given the above-mentioned approximation for the distribution of D, a z-value can be calculated corresponding to the two-sided p-value. Because the z-value is also equal to D/√(v(n)), which from the above calculation equals D*√{n*info(1) }, one can obtain info(1) as z2 /{ D2 *n }. Finally, the power of a study (with sample size n) to detect a difference d in the logit scale is 1 − Φ[1.96-d*√{n*info(1)} ].
Results
Median follow-up was 187 ± 110 weeks (38 to 435) from the date of diagnosis and 176 ± 108 weeks (56 to 431) from the date of listing. Technical success of chemoembolization was 100%. The patient distribution to time of transplant (or drop off) versus waiting time (calculated as described in the Statistical Analysis section) is shown in Fig. 1. Table 1 lists comparative variables for the control and test groups before removal of the patients without a propensity-comparable counterpart in the other group. The initial chemoembolization and nonchemoembolization groups were not matched for C-P distribution, tumor size, and AFP levels, necessitating the matching process described in the Statistical Analysis section. As previously mentioned, 13 chemoembolization patients did not have a comparable patient in the nonchemoembolization group with respect to the propensity score; the same held true for 9 patients in the nonchemoembolization group. In the subcohorts obtained after the removal of these patients, the chemoembolization and nonchemoembolization patients were found to be matched in all variables listed in Table 1.
DoTx or drop off for all patients in our study with HCC fulfilling Milan criteria. Among the 52 who were not treated with chemoembolization (top graph), 7 (13.5%) dropped off the transplant list (dark lines) at various times. In the subgroup that was treated with chemoembolization, (bottom graph), there was only 1 patient (3%) who dropped off the transplant list
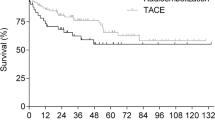
Median time from day of diagnosis (DoDx) to the day of listing (DoL) was 7 weeks for both the chemoembolization and nonchemoembolization group. In addition, there was no statistically significant difference in the time from DoDx to day of transplant (DoTx) or from DoL to DoTx between the chemoembolization and non chemoembolization group (Table 2). The overall Kaplan–Meier survival curve is shown in Figure 2.
Kaplan–Meier survival curves (time in months). There was no statistically significant survival difference between the nonchemoembolization (dashed) and chemoembolization (solid) groups. Survivals are from date of listing. Two-year survival for the nonchemoembolization group was 57.3% ± 7.1% and for the chemoembolization group was 76.0% ± 7.9% (p = 0.078 from DoL). Although the median survival has not been reached yet, it appears to be >5 years. Vertical bars are 95% confidence intervals
Drop-Off Risk
Seven of 52 patients (13.5%) from the nonchemoembolization group and 1 of 35 (3%) from the chemoembolization group dropped off the transplant list before receiving a transplant. After rendering the groups comparable as described above, s drop-off patients remained in the nonchemoembolization group, and 1 patient remained in the chemoembolization group. The risk of dropping off the transplant waiting list was greater for the nonchemoembolization group versus the chemoembolization group (15% vs. 3%, p = 0.040) (n = 65). Figure 3 shows the risk of dropping off the liver transplant waiting list at 4-month intervals for the patient group that was on the list for a minimum of that time. Median survival was not reached yet; therefore, we calculated and compared 2-year survival probabilities. Although the treatment group showed better 2-year survival, it was not statistically significant (Table 2). This was likely due to our study being underpowered for survival analysis. Finally, the Kaplan–Meier curve with end-point being dropping off the list is shown in Fig. 4.
Percent risk of dropping off the liver transplant list for patients with list time longer than (t) versus time spent on the transplant list (t). When all comparable patients from both groups were considered (t = 0, n = 65), the drop-off risk for the chemoembolization group was 3% compared with 15% for the nonchemoembolization group. The difference is statistically significant (p = 0.04). As waiting time gets longer, fewer patients are included in the risk calculation, and risk differences become statistically nonsignificant, i.e., p continuously increases
Sample Size and Power
By using the simulation distribution of the estimator D, we calculated p = 0.04. As described in the Sample Size Calculation section, we calculated the z-value corresponding to a two-sided p-value to be z = 2.05. Using also the observed values of the drop-off risks we calculated D = logit(0.15) − logit(0.03) = 1.74. (Observed sample sizes are n = 43[non-TACE] + 22[TACE] = 65).
Because the z-value = 1.74*√{65*info(1)} = 2.05, then info(1) = 0.02. Using the above-described approximation, the power of a study with sample size n to detect a difference d in the logit scale is 1 − Φ(1.96 − d*√(n*info1)). The overall risk of drop off is reported to be approximately 22% in the literature [7]. With 65 total patients (and the same ratio in TACE vs. non-TACE), the power to detect a risk decreased to 3% (as we did) would have been estimated as 1 − Φ(1.96 − [d = logit(.22) − logit(.3)]*√{65*info(1)} = 74%. Otherwise put, a sample size of 65 is needed to provide the statistical analysis with 74% probability of detecting that decrease in drop-off risk.
Discussion
The expected increase in cirrhosis and HCC (due to increasing incidence of hepatitis C virus and non-alcoholic steatohepatitis [26, 27]) in the foreseeable future, coupled with the lack of available livers for transplantation, will inevitably prolong the transplant waiting times for patients. It is important therefore to design treatment protocols that better select patients for liver transplantation and decrease the drop-off risk of such patients. Chemoembolization has been identified as a treatment strategy that could possibly accomplish both objectives [5, 10, 11, 16, 17].
Approximately half of the HCC patients who drop off the transplant list do so as a result of tumor progression [3]. Locoregional interventions may decrease the risk of drop off by prolonging time to disease progression, i.e., keeping the patient within Milan or San Francisco criteria. The post-MELD annual risk of drop out risk for patients with HCC is 20% to 27% [3, 28–30]. The median time to transplant is 64 days compared with only 46 days for all patients [3]. Indeed, the post-MELD era has placed HCC patients at a disadvantage, with HCC patients having a significantly longer time to transplant [31]. The introduction of exception-points for HCC has mitigated this problem and resulted in a decreased risk of drop off and increased posttransplant survival rates [31] for patients with HCC.
Many studies looking for potential benefits of chemoembolization in patients awaiting a liver transplant for HCC are inconclusive or contradictory, partly because of lack of stratification. For example, chemoembolization cannot reasonably be expected to affect the drop-off rate of patients who are high on the transplant list because the waiting time is short. Similarly, chemoembolization is of doubtful benefit if a patient is on the transplant list for an extended period of time due to high risk of disease progression. In contrast, studies in which patients are stratified appropriately have shown that chemoembolization indeed benefits such patients.
Literature suggests chemoembolization improves posttransplant survival but only if the transplant waiting time is between 4 and 9 months [20, 22]. Furthermore, chemoembolization appears to improve the 5-year posttransplant survival for patients whose HCC is >2 cm (vs. <2 cm; 78% vs. 37% respectively; p = 0.016) [11], presumably because they are closer to the Milan criteria limit for size. Response to chemoembolization also appears to be a positive predictive factor for posttransplant survival. Patients who demonstrate EASL or RECIST response show better posttransplant survival compared with their counterparts who show no response to chemoembolization (1-, 2-, 5-year survival = 89%, 85%, 85% versus 89%, 77%, 64% versus 69%, 51%, and 51%) [9]. Explants show a rate of complete tumor necrosis between 28% and 46% after chemoembolization [8, 10, 11, 16]. Another study showed that response to chemoembolization is a positive predictor, with a 5-year survival rate of 94% versus only 35% for those whose disease progressed while on chemoembolization treatment [5, 6, 17, 21].
Adding to the above-mentioned possible benefits of chemoembolization, our study concludes that it significantly decreases the risk of dropping off the liver transplant list for patients with HCC. This risk decrease from 15% to 3% represents an absolute risk decrease of nearly 80% (p = 0.04). Figure 3 shows the risk decrease while on the liver transplant list. Not all patients, however, are expected to benefit equally. On one end of the spectrum are patients with high MELD scores and blood types that make their transplant waiting time <4 months. Such patients would likely not benefit from chemoembolization. Similarly, patients with living related donors should not risk delaying the transplant time by being treated with chemoembolization. On the other end of the spectrum are patients with low MELD scores and/or other factors that will likely prolong their waiting time. According to Figure 3, patients in our study waiting >20 months for a liver transplant were unlikely to benefit from chemoembolization. Although the exact time period was not accurately defined, it follows from our data as well as the literature mentioned above that the benefit of chemoembolization is lost if a patient has to wait too long to receive a liver transplant. The benefit in question, however, is the decrease in the risk of dropping off the waiting list. It should be remembered that chemoembolization does confer a survival advantage to nonresectable patients, which should still be applicable to those who drop off the transplant list.
Differences in survival (whether calculated from DoDx, DoL, or DoTx) between the treatment and nontreatment group are not statistically significant. However, the treatment group had a strong tendency toward longer survival despite the fact that patients in that group had poorer survival predictors (i.e., more tumors and larger tumors). The lack of statistically significant difference is likely due to lack of power.
Limitations
There are certain limitations to our study. It is a retrospective study and may suffer from biases beyond our ability to control. Another shortcoming is the lack of blood-group stratification. Although blood group is an important predictor for how long a patient awaits on the liver transplant list, we believe this was not a significant bias in our study for two reasons. First, none of the other variables are correlated with blood type and therefore we expect the blood types to match between the two groups. This is of course not certain. Second, and more importantly, the waiting time between the two groups was not different; therefore, even if the two groups were not matched for blood type, it should not have had an effect on the drop-off risk. The final limitation was that we included a small number of patients from 2001 (n = 7), which was before the use of MELD for allocation. We do not expect this to have had an impact on our conclusion because of the small number of patients and the fact that MELD scores were matched between groups.
A prospective, randomized study is required to define the exact benefit of chemoembolization. Based both on related literature and our data, patients expected to be on the liver transplant wait list >4 months [20, 23] should be considered for such treatment.
References
Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F et al (1996) Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 334:693–699
Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A et al (2001) Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 33:1394–1403
Pelletier SJ, Fu S, Thyagarajan V, Romero-Marrero C, Batheja MJ, Punch JD et al (2009) An intention-to-treat analysis of liver transplantation for hepatocellular carcinoma using organ procurement transplant network data. Liver Transpl 15(8):859–868
Stockland AH, Walser EM, Paz-Fumagalli R, McKinney JM, Mayrger GR (2007) Preoperative chemoembolization in patients with hepatocellular carcinoma undergoing liver transplantation: influence of emergent versus elective procedures on patient survival and tumor recurrence rate. Cardiovasc Intervent Radiol 30:888–893
Obed A, Beham A, Püllmann K, Becker H, Schlitt HJ, Lorf T (2007) Patients without hepatocellular carcinoma progression after transarterial chemoembolization benefit from liver transplantation. World J Gastroenterol 13(5):761–767
Kim DY, Moon-Choi S, Lee JH, Koh KC, Paik SW, Yoo BC et al (2006) Milan criteria are useful predictors for favorable outcomes in hepatocellular carcinoma patients undergoing liver transplantation after transarterial chemoembolization. World J Gastroenterol 12(43):6992–6997
Wiesner R, Freeman RB, Mulligan DC (2004) Liver transplantation for hepatocellular cancer: the impact of the MELD allocation policy. Gastroenterology 127(Suppl):S261–S267
Chapman WC, Majella-Doyle MB, Stuart JE, Vachharajani N, Crippin JS, Anderson CD et al (2008) Outcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantation. Ann Surg 248(4):617–625
Dharancy S, Boitard J, Decaens T, Sergent G, Boleslawski E, Duvoux C et al. (2007) Comparison of two techniques of transarterial chemoembolization before liver transplantation for hepatocellular carcinoma: a case–control study. Liver Transplant 13:665–671
Millonig G, Graziadei IW, Freund MC, Jaschke W, Stadlmann S, Ladurner R et al (2007) Response to preoperative chemoembolization correlates with outcome after liver transplantation in patients with hepatocellular carcinoma. Liver Transplant 13:272–279
Bharat A, Brown DB, Crippin JS, Gould JE, Lowell JA, Shenoy S et al (2006) Pre-liver transplantation locoregional adjuvant therapy for hepatocellular carcinoma as a strategy to improve long-term survival. J Am Coll Surg 203:411–420
http://optn.transplant.hrsa.gov/latestData/rptData.asp. Accessed 1 Nov 2010
Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT et al (2002) Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 35(5):1164–1171
Llovet JM, Bruix J (2003) Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 37(2):429–442
Camma C, Schepis F, Orlando A, Albanese M, Shahied L, Trevisani F et al (2002) Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology 224(1):47–54
Graziadei IW, Sandmueller H, Waldenberger P, Koenigsrainer A, Nachbaur K, Jaschke W et al (2003) Chemoembolization followed by liver transplantation for hepatocellular carcinoma impedes tumor progression while on the waiting list and leads to excellent outcome. Liver Transplant 9(6):557–563
Otto G, Herber S, Heise M, Lohse AW, Monch C, Bittinger F et al (2006) Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transplant 12:1260–1267
Sotiropoulos GC, Malago M, Molmenti EP, Radtke A, Brokalaki EI, Nadalin S et al (2005) Disease course after liver transplantation for hepatocellular carcinoma in patients with complete tumor necrosis in liver explants after performance of bridging treatments. Eur J Med Res 10(12):539–542
Saborido BP, Meneu JC, Moreno E, García I, Moreno A, Fundora Y (2005) Is transarterial chemoembolization necessary before liver transplantation for hepatocellular carcinoma? Am J Surg 190:383–387
Decaens T, Roudot-Thoraval F, Bresson-Hadni S, Meyer C, Gugenheim J, Durand F et al (2005) Impact of pretransplantation transarterial chemoembolization on survival and recurrence after liver transplantation for hepatocellular carcinoma. Liver Transplant 11:767–775
Otto G, Heise M, Moench C, Herber S, Bittinger F, Schuchmann M et al (2007) Transarterial chemoembolization before liver transplantation in 60 patients with hepatocellular carcinoma. Transplant Proc 39:537–539
Zimmerman MA, Trotter JF, Wachs M, Bak T, Campsen J, Wright F et al (2007) Predictors of long-term outcome following liver transplantation for hepatocellular carcinoma: a single-center experience. Transplant Int 20(9):747–753
Aloia TA, Adam R, Samuel D, Azoulay D, Castaing DA (2007) Decision analysis model identifies the interval of efficacy for transarterial chemoembolization (TACE) in cirrhotic patients with hepatocellular carcinoma awaiting liver transplantation. J Gastrointest Surg 11:1328–1332
Rosenbaum PR, Rubin DB (1983) The central role of the propensity score in observation studies for causal effects. Biometrika 79:516–524
Rosenbaum PR (1984) Conditional permutation tests and the propensity score in observational studies. J Am Stat Assoc 79:565–574
Marchesini G, Moscatiello S, Di Domizio S, Forlani G (2008) Obesity-associated liver disease. J Clin Endocrinol Metab 93(11 Suppl 1):S74–S80
Caldwell SH, Crespo DM, Kang HS, Al-Osaimi AM (2004) Obesity and hepatocellular carcinoma. Gastroenterology 127(5 Suppl 1):S97–S103
Kim WR, Therneau TM, Benson JT, Kremers WK, Rosen CB, Gores GJ et al (2006) Deaths on the liver transplant waiting list: an analysis of competing risks. Hepatology 43(2):345–351
Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT et al (2008) Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med 359(10):1018–1026
Gotthardt D, Weiss KH, Baumgartner M, Zahn A, Stremmel W, Schmidt J et al (2009) Limitations of the MELD score in predicting mortality or need for removal from waiting list in patients awaiting liver transplantation. BMC Gastroenterol 9:72
Ioannou G, Perkins JD, Carithers RL Jr (2008) Liver transplantation for hepatocellular carcinoma: impact of the MELD allocation system and predictors of survival. Gastroenterology 134(5):1342–1351
Acknowledgements
Dr. Jean-Francois Geschwind is a consultant for Biosphere Medical, Biocompatibles, Bayer Healthcare and has grant support from Biospheres, Biocompatibles, MDS Nordion, Genentech, Philips Medical, Bayer Healthcare. Contect Vision, Gideon.
Conflict of interest
The other authors declare they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Frangakis, C., Geschwind, JF., Kim, D. et al. Chemoembolization Decreases Drop-Off Risk of Hepatocellular Carcinoma Patients on the Liver Transplant List. Cardiovasc Intervent Radiol 34, 1254–1261 (2011). https://doi.org/10.1007/s00270-010-0077-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-010-0077-7