Abstract
Purpose
This study was designed to clarify the advantages of biodegradable stents in terms of mucosal reaction and biodegradation after placement. We designed a biodegradable stent and assessed stent degradation and changes in the normal bile ducts of dogs.
Methods
The biodegradable stent is a balloon-expandable Z stent consisting of poly-l-lactic acid (PLLA) with a diameter of 6 mm and a length of 15 mm. We assessed four groups of three beagle dogs each at 1, 3, 6, and 9 months of follow-up. After evaluating stent migration by radiography and stent and bile duct patency by cholangiography, the dogs were sacrificed to remove the bile duct together with the stent. The bile duct lumen was examined macroscopically and histologically, and the stent degradation was examined macroscopically and by scanning electron microscopy (SEM).
Results
Bile duct obstruction was absent and none of the stents migrated. Macroscopic evaluation showed moderate endothelial proliferation in the bile ducts at the implant sites at 3 and 6 months and a slight change at 9 months. Slight mononuclear cell infiltration was histologically identified at all time points and epithelial hyperplasia that was moderate at 3 months was reduced to slight at 6 and 9 months. Stent degradation was macroscopically evident in all animals at 9 months and was proven by SEM in two dogs at 6 months and in all of them at 9 months.
Conclusions
Our results suggest that PLLA bioabsorbable stents seems to be useful for implantation in the biliary system with further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Because the advantages of expandable metallic stents include reliable internal drainage with longer-term patency than plastic tube stent, metallic stent placement is realized as an effective treatment option in malignant biliary obstruction. However, metallic stent is generally not indicated in patients with benign biliary strictures with expected long-term survival because stent obstruction due to intimal hyperplasia and biliary sludge as well as cholangitis after stent implantation and in addition, impossibility to remove after implantation. On the other hand, biodegradable stent might be able to address the problems associated with metallic stents and replace them as treatment for benign biliary strictures. However, tissue reactions and stent degradation characteristics after placement in the bile duct have not been sufficiently investigated [1]. In this study, we designed and inserted balloon-expandable, biodegradable poly-l-lactic acid (PLLA) Z stents into the normal dog bile duct to clarify their effects on mucosal reaction and biodegradation without removal. We also macroscopically and histologically examined bile duct reactions to the stents.
Materials and Methods
Biodegradable Stent and Delivery System
The biodegradable stent consists of 0.8 mm PLLA fibers with six bends in a zigzag configuration to form a cylinder, similar to that of a metallic Z stent, with a length and diameter of 15 and 6 mm, respectively. The stents were configured with a platinum marker for X-ray visualization and sterilized under γ radiation (Fig. 1). Each stent was mounted onto a balloon catheter (length and diameter, 20 and 6 mm, respectively) and inserted through a 7-Fr sheath as the delivery system (Fig. 2).
Animal Care and Stent Deployment
This study proceeded in accordance with standard guidelines and local regulations as specified by the animal care committee. Twelve male beagle dogs (age, 8 months; weight, 12–13.3 kg; mean, 12.6 kg) were anesthetized with intravenous ketamine and intubated with an endotracheal tube to secure the airway. The gall bladder was exposed through a midline abdominal incision and then punctured with a 19-gauge elaster needle and injected with twofold diluted contrast agent (Urograffin) for cholangiography (Fig. 3A). Contrast agent was injected until the duodenum was visualized, and the position of the papilla was confirmed. The opening of the papilla was visualized through a small incision in the duodenum opposite the papilla, and a 5-Fr catheter was inserted in retrograde fashion into the common bile duct. A 0.035-inch guidewire was inserted into the catheter, and then the delivery system was introduced over the guidewire and positioned in the common bile duct (Fig. 3B). The implantation site was determined, the 7-Fr sheath was pulled backwards, the stent was released, the balloon was expanded, and the stent was placed in the bile duct wall (Fig. 3C). Thereafter, contrast agent was injected from the gallbladder to check the position of the stent (Fig. 3D). The catheter was removed, the duodenal wall was sutured, and the abdomen was closed.
Follow-up Procedures
The 12 dogs were divided into 4 groups of 3 based on follow-up at 1, 3, 6, and 9 months. A laparotomy was performed under general anesthesia at each time point, and the position of the stent marker was confirmed by plain radiography. Direct cholangiography was performed by gallbladder puncture to evaluate stent migration as well as the patency of the stent and bile duct. The animals were sacrificed with KCl, and then the liver, gallbladder, bile duct, and duodenum were removed en bloc.
Macroscopic and Histological Assessments
Macroscopic Examination
Stents were visually inspected for damage or degradation through a longitudinal incision then removed to observe the bile duct lumen.
Macroscopic findings regarding endothelial redness, endothelial proliferation, and stent embedding were analyzed. Findings showing a white and transient tissue reaction, such as a hyperplasia, were defined as endothelial proliferation. Endothelial proliferation and stent embedding were classified as absent (-), slight (+), moderate (++), and severe (+++) according to the degree of changes.
Histological Examination
The bile duct was fixed in formalin, stained with hematoxylin and eosin (HE), and examined by microscopy. Findings of mononuclear cell infiltration, epithelial hyperplasia, and epithelial necrosis were examined. Mononuclear cell infiltration and epithelial hyperplasia also were graded as absent (-), slight (+), moderate (++), and severe (+++). The surface and cross-section of biodegradable stent were examined by scanning electron microscopy (SEM).
Results
All stents were positioned in the bile duct without complications.
-
1)
Evaluation by plain radiography
At 1, 3, and 6 months, all stents in nine dogs were identified without any migration of the platinum marker. However, some of the platinum marker disappeared with biodegradation of the stent in three dogs at 9 months.
-
2)
Evaluation by cholangiography
Cholangiography revealed the passage of contrast agent without evidence of bile duct obstruction in all 12 dogs. The stent marker was visualized outwards from the bile duct lumen by cholangiography in the bile duct wall of one, two, and one of the dogs at 3, 6, and 9 months (no. 6, 7, 8, and 11), respectively (Fig. 4). Those findings suggest that the stents might be embedded in the bile duct wall, which were realized on the following macroscopic findings. Narrowing of bile duct was not evident in these four dogs, and macroscopic examination of the bile duct lumen confirmed partial or total embedding of the stent in the bile duct wall.
-
3)
Bile duct changes
-
(a)
Macroscopic findings (Fig. 5; Table 1)
Endothelial redness in the bile duct at the stent implant site was absent (-) in all dogs (Fig. 5A). Endothelial proliferation (Fig. 5B and D) was recognized at the stent implant site in 5 (no. 4, 5, 9, 10, and 12) of 12 dogs, of which 2 (no. 4 and 5) were moderate (++) at 3 months, 1 (No. 9) was slight (+) at 6 months, and 2 (No. 10 and 12) were slight (+) at 9 months. The stents were easily removed without being embedded in the bile duct wall in 7 (no. 1, 2, 3, 4, 5, 9, and 12) of 12 dogs. On the other hand, in the three dogs (no. 6-8) in which no endothelial proliferation was identified at 3 and 6 months, the stents were partially or totally embedded in the bile duct wall and could not be removed (Fig. 5C). In addition, in one dog (no. 10) with slight (+) endothelial proliferation at 9 months and in one dog (no. 11) without endothelial proliferation at 9 months, a piece of biodegraded stent was embedded in the bile duct wall (Table 1).
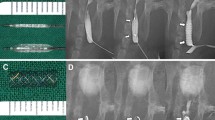
Fig. 4 Follow-up cholangiography. A Biliary obstruction, stent migration and bile duct narrowing are absent. Platinum marker is depicted outwards from bile duct lumen (arrowhead). B Contrast agent passes easily through stent, but CBD is dilated at proximal side of stent (arrow). Stent is not dislocated and marker is confirmed outwards from bile duct lumen (arrowhead)
Fig. 5 Macroscopic examination of stent placed in bile duct. A Bile duct lumen in all 12 dogs is smooth with no signs of endothelial redness. B Moderate (++) endothelial proliferation recognized at stent implant site at 3 months (no. 4 and 5). Stent was removed easily and trace remained in bile duct wall (arrow). C Stent totally embedded in bile duct wall (arrowhead) at 6 months was not removable (no. 7 and 8). D Endothelial proliferation at 9 months (no. 10 and 12) is mild compared with that at 3 months. Stents have been absorbed and have disappeared
Table 1 Macroscopic findings of bile duct -
(b)
Histological findings (Fig. 6; Table 2)
Fig. 6 Histological examination of stent placed in bile duct. A Slight (+) mononuclear cell infiltration and epithelial necrosis at 1 month (no. 1 and 2). B Slight (+) mononuclear cell infiltration and moderate (++) epithelial hyperplasia at 3 months (no. 4 and 6). C Slight (+) mononuclear cell infiltration; epithelial hyperplasia has decreased from moderate to slight (+) at 6 months (no. 7–9). D Slight (+) mononuclear cell infiltration; epithelial hyperplasia has slightly decreased at 9 months (no. 11 and 12), compared with 6 months
Table 2 Histological findings of bile ducts Mononuclear cell infiltration (Figs. 6A–D) was slight (+) in the region of stent placement in 11 dogs except for 1 (no. 10) at 9 months. Epithelial hyperplasia was found in 9 dogs, but not in 3 (no. 1–3) at 1 month. Epithelial hyperplasia was moderate (++) in 2 dogs (No. 4 and 6) at 3 months. Slight (+) epithelial hyperplasia (Fig. 6B) was recognized in 1 dog (No. 5) at 3 months and in 6 (no. 7–12) at 6 and 9 months. Epithelial hyperplasia was reduced at 6 and 9 months compared with that at 3 months (Table 2). Moreover, epithelial hyperplasia tended to diminish at 9 months compared with 6 months (Fig. 6C, D). In addition, slight (+) epithelial necrosis (Fig. 6A) was recognized in 2 dogs (no. 1 and 2) at 1 month (Table 2).
-
(c)
Analysis of removed stent by SEM (Fig. 7).
Fig. 7 We found no macroscopic evidence of stent degradation in nine dogs at 1, 3, and 6 months. Minimal cracking of the stent surface was revealed by SEM in two dogs (no. 8 and 9) at 6 months. Macroscopic examination of the stents removed from three dogs (no. 10–12) at 9 months, indicated moderate fragmentation and biodegradation in all of them, and SEM revealed partial rupture or cavitation both at the surface and inside the stents.
-
(a)
Discussion
Metallic stents have several advantages, such as reliable internal drainage, long-term patency, and applicability to various types of obstructive lesions, and their usefulness for malignant biliary obstruction is almost established [2]. However, metallic stents have the disadvantages of complications, such as obstruction due to epithelial hyperplasia and biliary sludge causing cholangitis after placement. In addition, epithelial formation and embedding into the bile duct walls after implantation makes metallic stents difficult to remove. Epithelial hyperplasia or biliary sludge frequently obstructs metallic stents over the long-term, which causes difficulties with placement when considering treatment for benign biliary obstruction. On the other hand, some reports show that the stent made of biodegradable materials is useful for urological and cardiovascular conditions [3–5]. Medical devices have incorporated biodegradable materials since 1966 when sutures were made of polylactic acid [6]. Biodegradable materials have high biocompatibility, and clinical applications as well as an experimental study of stents in the bile duct have been reported [1]. Although biodegradable materials have high biocompatibility, experimental study about the bile duct stent has been limited. Biodegradable stents are less likely to cause epithelial proliferation and form biofilms. In addition, biodegradation over time means that the stents do not require removal and thus will cause fewer artifacts on images. Biodegradable stents have many features that seem superior to metallic stents, but their clinical applicability remains to be investigated in detail. We, therefore, designed a biodegradable stent for deployment in the normal bile ducts of dogs to investigate biocompatibility and degradation characteristics.
Our findings revealed no obstruction at the sites of all implanted PLLA stents, some embedding of stents in the bile duct wall, and no migration. A macroscopic examination showed no endothelial redness in the bile duct walls. Although endothelial proliferation of the bile duct wall was recognized in two, one, and two dogs at 3, 6, and 9 months, respectively, the degree peaked at 3 months and diminished after 6 and 9 months. The endothelial proliferation was probably due to bacterial aggregation with glycocalyx or bile acid deposition [7]. No bile duct obstruction was present in any dog with these changes, but further bacterial growth could cause biofilm formation or cholangitis, with subsequent bile duct obstruction, which could be prevented by impregnating stents with antibiotics [8].
Tamai et al. [3] found that vascular intima started to cover PLLA stents implanted into coronary arteries at 6 months. The stent itself or intimal injury at the stent implant site might cause thrombosis and subsequent intimal formation in blood vessels. On the other hand, Ginsberg et al. found no embedding of PLLA stents in the epithelium of the porcine bile duct at 12 months after implantation [1]. We found embedded stents in the bile duct walls of one, two, and two dogs at 3, 6, and 9 months, and we speculate that the cause was inflammation at the point where the stent contacted the bile duct lumen. Histological examination showed slight mononuclear cell infiltration in the bile duct wall and epithelial hyperplasia was evident in all dogs except in the 1-month group, but the degree decreased from moderate to slight over time. Others have not found epithelial hyperplasia or mononuclear cell infiltration [1, 9, 10], but we found slight histological changes induced by our PLLA stent compared with those induced by metallic stents.
Our stents maintained their configuration for up to 6 months and fragmentation occurred in all dogs at 9 months, indicating that biodegradation starts between 6 and 9 months. A bougie must be effective for at least 6 months to improve biliary obstruction [11]. The period until biodegradation starts seemed satisfactory for biliary application, but biodegradation processes and tissue reactions differ according to material. Other possible materials for biodegradable stents include poly-L/D-lactic acid (PLA96) and polylactide and glycolic acid (PLGA). Although the properties of PLA96 and PLGA are similar to those of PLLA, PLGA biodegrades faster than PLLA, and PLA96 causes more tissue reaction than the others [4, 6, 10, 12]. Therefore, PLLA seems to be highly biocompatible and might thus be a suitable construction material for bile duct stents.
Our stent was constructed as a zigzag form with a platinum marker to confirm its position under fluoroscopy. Stent shape has not been defined and improvements in visibility, such as adding barium have been described [1, 13]. However, we believe that further examination is required to develop self-expandable stents with a low-profile delivery system [14].
Conclusions
Our PLLA biodegradable stent might be useful for implantation into the benign biliary obstruction since tissue reactions were minimal, configuration was maintained for 6 months and it was partially biodegraded by 9 months in the normal dog bile duct. However, we think that further investigation with longer follow-up is suitable to clear this point.
References
Ginsberg G, Cope C, Shah J et al (2003) In vivo evaluation of a new bioabsorbable self-expanding biliary stent. Gastrointest Endosc 58(5):777–784
Yoshioka T, Sakaguchi H, Yoshimura H et al (1990) Expandable metallic biliary endoprostheses: preliminary clinical evaluation. Radiology 177(1):253–257
Tamai H, Igaki K, Kyo E et al (2000) Initial and 6-month results of biodegradable poly-l-lactic acid coronary stents in humans. Circulation 102(4):399–404
Lumiaho J, Heino A, Pietiläinen T et al (2000) The morphological, in situ effects of a self-reinforced bioabsorbable polylactide ureteric stent: an experimental study. J Urol 164(4):1360–1363
Talja M, Multanen M, Välimaa T et al (2002) Bioabsorbable SR-PLGA horn stent after antegrade endopyelotomy: a case report. J Endourol 16(5):299–302
Laaksovirta S, Talja M, Välimaa T et al (2001) Expansion and bioabsorption of the self-reinforced lactic and glycolic acid copolymer prostatic spiral stent. J Urol 166(3):919–922
Unverdorben M, Spielberger A, Schywalsky M et al (2002) A polyhydroxybutyrate biodegradable stent: preliminary experience in the rabbit. Cardiovasc Intervent Radiol 25(2):127–132
Multanen M, Talja M, Tammela TL et al (2001) Biocompatibility of silver nitrate and ofloxacine coated bioabsorbable SR-PLLA rods. Urol Res 29(2):113–117
Saito Y, Minami K, Kobayashi M et al (2002) New tubular bioabsorbable knitted airway stent: biocompatibility and mechanical strength. J Thorac Cardiovasc Surg 123(1):161–167
Korpela A, Aarnio P, Sariola H et al (1998) Comparison of tissue reactions in the tracheal mucosa surrounding a bioabsorbable and silicone airway stents. Ann Thorac Surg 66(5):1772–1776
Born P, Rösch T, Brühl K et al (1999) Long-term results of endoscopic and percutaneous transhepatic treatment of benign biliary strictures. Endoscopy 31(9):725–731
Talja M, Multanen M, Välimaa T et al (2002) Bioabsorbable SR-PLGA horn stent after antegrade endopyelotomy: a case report. J Endourol 16(5):299–302
Isotalo T, Alarakkola E, Talja M et al (1999) Biocompatibility testing of a new bioabsorbable x-ray positive SR-PLA 96/4 urethral stent. J Urol 162(5):1764–1767
Isotalo T, Talja M, Välimaa T et al (2002) A bioabsorbable self expandable, self reinforced poly L lactic acid urethral stent for recurrent urethral strictures long term results. J Endourol 16(10):759–762
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamamoto, K., Yoshioka, T., Furuichi, K. et al. Experimental Study of Poly-l-Lactic Acid Biodegradable Stents in Normal Canine Bile Ducts. Cardiovasc Intervent Radiol 34, 601–608 (2011). https://doi.org/10.1007/s00270-010-0045-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-010-0045-2