Abstract
The purpose of this study was to investigate the feasibility of a flat-detector C-arm–guided radiographic technique (cone-beam computed tomography [CBCT]) for percutaneous radiologic gastrostomy (PRG) insertion. Eighteen patients (13 men and 5 women; mean age 62 years) in whom percutaneous endoscopic gastrostomy (PEG) had failed underwent CBCT-guided PRG insertion. PEG failure or unsuitability was caused by upper gastrointestinal tract obstruction in all cases. Indications for gastrostomy were esophageal and head and neck malignancies, respectively. Before the PRG procedure, initial C-arm CBCT scans were acquired. Three- and 2-dimensional soft-tissue reconstructions of the epigastrium region were generated on a dedicated workstation. Subsequently, gastropexy was performed with T-fasteners after CBCT-guided puncture of the stomach bubble, followed by insertion of an 14F balloon-retained catheter through a peel-away introducer. Puncture of the stomach bubble and PRG insertion was technically successful in all patients without alteration of the epigastric region. There was no malpositioning of the tube or other major periprocedural complications. In 2 patients, minor complications occurred during the first 30 days of follow-up (PRG malfunction: n = 1; slight infection: n = 1). Late complications, which were mainly tube disturbances, were observed in 2 patients. The mean follow-up time was 212 days. CBCT-guided PRG is a safe, well-tolerated, and successful method of gastrostomy insertion in patients in whom endoscopic gastrostomy is not feasible. CBCT provides detailed imaging of the soft tissue and surrounding structures of the epigastric region in one diagnostic tour and thus significantly improves the planning of PRG procedures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Percutaneous radiologic gastrostomy (PRG) is a well-tolerated procedure that provides essential nutritional support in patients with an obstructed enteral passage caused by advanced tumours of the pharynx, larynx, or oesophagus [1]. Compared with percutaneous endoscopic gastrostomy (PEG), PRG has shown lower morbidity and mortality rates; furthermore, significantly fewer major complications have been reported with PRG [2, 3].
The percutaneous method may incorporate a number of safety measures, including barium opacification of the colon and air insufflation of the stomach, which provide a fluoroscopic image to the interventional radiologist [4, 5]. Furthermore, preprocedural sonographic examinations of the abdomen for left liver edge localization may be used [1]. In most cases, the access site is localized by way of fluoroscopic examination of the distended air-insufflated stomach after nasogastric tube placement [4–6]. In a few patients, however, technical difficulties require additional preinterventional CT guidance for successful stomach access [5]. In these patients, preprocedural CT resulted in prolonged procedural time duration as well as longer fluoroscopy times necessary for the procedure [7, 8]. To overcome these problems, CBCT-guided PRG insertion was performed at our institute in 18 consecutive patients who were not suitable for endoscopic approach.
Materials and Methods
CBCT-guided PRG placement for long term-enteral nutrition was performed in 18 consecutive patients (13 men and 5 women; age range 47 to 86 years [mean 62]) with head and neck malignancies (carcinomas of the larynx: n = 3; pharynx: n = 11; esophagus/paraesophageal: n = 2; other: n = 2). Thirteen patients underwent percutaneous radiation therapy. All of the patients referred to our institute had malignant strictures of the upper gastrointestinal tract, which are considered a contraindication to endoscopic approach, or had a history of previous unsuccessful PEG attempts.
Patients fasted overnight before the procedure, and informed consent was obtained. For appropriate air distension of the stomach, a nasogastric tube was placed the evening before the procedure in 11 patients. In 7 patients with severe obstruction of the oropharynx or esophagus, the obstructions were passed using a 5.0F hydrophilic-coated angiographic vertebralis catheter (William Cook Europe, Bjaeverskov, Denmark) after fluoroscopic guidance using a .035-inch nitinol hydrophilic guidewire (Terumo Europe, Leuven, Belgium). After correct intragastric placement, the wire was removed, and approximately 500 ml air was insufflated for appropriate stomach distension. For each patient, initial C-arm CBCT was performed while his or her respiration was stopped (Fig. 1).
Non–contrast-enhanced CBCT was acquired with the use of a commercially available 30 × 40–cm flat-panel angiography system (Allura FD20; Philips Medical Systems, Best, The Netherlands). The acquisition used for the PRG procedure was based on a soft tissue–reconstruction technique with a C-arm movement range of 240º with acquisition of 120 images (acquisition time 8 seconds; matrix size 1024 × 1024; depth 14 bits). The acquired rotational frames were automatically transferred to a 3DRA workstation (XtraVision, Dell Computer, Round Rock, TX) and reconstructed. The workstation is fully integrated with the acquisition system to allow real-time creation of three-dimensional (3D) reconstructions and CT-like two-dimensional images. In this process, the soft-tissue reconstructions are created within 90 seconds. Implementation of the 3D reconstruction is directly coupled to the C-arm geometry, allowing for synchronized movement of the system geometry and the 3D reconstruction itself as well as real-time superimposition of the fluoroscopy frames onto the 3D reconstructions (i.e., 3D roadmapping).
After obtaining the CBCT run to exclude interposition of the left liver lobe or colon, the needle path was planned, and the skin entry point was marked. Disinfection was performed and local anaesthesia administered before puncture. We punctured the anterior stomach wall under fluoroscopy using 3D roadmapping guidance with an 18-gauge needle (Fig. 2). Once aspiration of air or contrast medium injection confirmed correct intragastric positioning, gastropexy was achieved with the use of two (n = 2 patients) and three (n = 16 patients) T-fasteners inserted around the site of the proposed gastrostomy. Subsequently, an 18-gauge needle was used to puncture the centre point of the gastropexy. Afterward, a 0.035-inch Amplatz extra-stiff guidewire was coiled into the stomach. The tract was dilated to accomodate a 15F peel-away sheath by passing increasingly larger dilators over the guidewire. Finally, a 14F Russell balloon-gastrostomy tube (William Cook Europe, Bjaeverskov, Denmark) was inserted over the guidewire and through the sheath into the stomach. The sheath was peeled away, and the balloon was blocked to avoid dislocation. A test injection of contrast medium was performed to check gastrostomy tube position in all patients at the end of each procedure (Fig. 3).
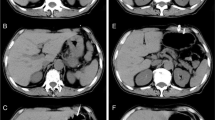
CBCT. A Axial reconstruction and B saggital reconstruction during needle placement. Puncture of the anterior stomach wall (triangle) and intragastral guidewire placement (star) was achieved without laceration of the colon and left liver lobe. C Corresponding fluoroscopic image of the upper abdomen (lateral view) shows linked needle tract during anterior gastric wall penetration
Patients remained fasting for 12 to 24 hours after the procedure. Single-shot antibiotic prophylaxis was administered routinely. Feeding commenced the next day after confirmation of correct tube placement and patency by fluoroscopic re-examination. All T-fasteners were removed within 7 days after gastrostomy insertion. Routine tube exchange was planned after 3 months. The tubes were routinely flushed after feeding to avoid blockage. Follow-up lasted until death or until removal of the tube.
Results
CBCT-guided percutaneous radiologic gastrostomy was technically successful in all patients. Two independent radiologists scored the overall image quality of CBCT reconstructions and roadmapping to be excellent in all patients such that the procedure could be safely performed. Median fluoroscopy time was 4,32 minutes (range 2,01 to 7,06). The mean cumulative radiation exposure was 31,232 mGy/cm2 (dose-area product range 11,504 to 67,925). Median procedure time from arrival at the angiography suite to completion of the entire procedure was 30,1 minutes (range 23,14 to 47,39).
All patients were followed-up retrospectively (mean follow-up 212 days [range 47 to 485]). There were no major complications or deaths in the first 30 days after gastrostomy insertion. Minor complications in the first 30 days after PRG were recorded in two patients, one with periostomal inflammation and another with defect tube attachment; however, neither abscess nor peritonitis developed. Furthermore, no major complications were noted within the follow-up period. Two patients died as a result of their underlying disease on postprocedure days 47 and 60, respectively. Minor complications occurred after the first 30 days in two patients with tube dislodgment and blockage, respectively. Both were successfully treated by replacing the tube with a new one. Seven patients had the gastrostomy removed after clinical improvement of their underlying disease.
Discussion
CBCT is a new technology used to obtain CT-like images using a C-arm angiography system that rotates around the patient [9–12]. A C-arm system equipped with a large flat-panel detector can obtain high-quality images and a large field of view compared with the image-intensified system [11]. This technology has been recently introduced into several interventional procedures [9–13]. CT-like images can easily be obtained with CBCT-during an interventional procedure simply by lifting the patient’s hands without causing other patient movement [11]. Soft-tissue scanning performed with the angiogaphy C-arm provides higher spatial resolution than corresponding CT scans as well as satisfactory contrast resolution, which allows for clinically justified soft-tissue differentiation [10]. The ability to couple the soft-tissue imaging information obtained from C-arm CBCT- to real-time fluoroscopic procedural imaging represents a significant advancement of the technology and provides the angiography suite with enhanced capabilities [12]. This is particularly important with regard to PRG insertion because potential complications, including colonic perforation or laceration of left liver lobe, can be avoided using this technique. In addition, CBCT-can be obtained within seconds in the angiography suite without a time lag between preprocedural conventional planning multislice spiral CT and the PRG insertion procedure. No additional complex technical equipment or time-consuming transportation to a different department is necessary.
PRG is usually performed in retrograde fashion under fluoroscopic guidance through the anterior abdominal wall, with the patient under local anaesthesia, after inflating the stomach with air or carbon dioxide by way of a nasogastric tube [1, 2]. When a nasogastric tube cannot be passed because of an obstructive lesion, distension is achieved by administering effervescent granules or by passing a fine-bored catheter under fluoroscopic guidance or a fine-bored needle (22G) percutaneously under CT or ultrasound guidance. Glucagon or buthylscopolamine can be given before gastric insufflation to decrease gastric motility [2]. Nevertheless, liver laceration and exsanguination [14], liver abscess, and persisting fistula [15] have been reported with transhepatic placement of PRG [16].
In our experience, projective fluoroscopy is sufficient to control PRG procedures in the majority of patients, although sonography proved valuable for liver position and depth calculation to the stomach. Occasionally it is difficult to adequately define the relation between the stomach and left liver lobe and the interpositioned colon. Factors that contribute to complications during the PRG procedure usually are anatomic problems such as hepatomegaly, an enlarged or irregular left liver lobe, previous surgery (e.g., Billroth II gastroenterostomy), ascites, or interposition of the colon between the stomach and the anterior abdominal wall requiring detailed preinterventional information concerning the approach [1, 2, 4]. In addition, patients with portal hypertension and gastric varices have an increased risk of peritoneal hemorrhage [8]. Therefore, routine use of ultrasound or CT evaluation before undertaking the procedure has been advocated by some investigators to ascertain safe placement of the catheter [1, 14, 16].
Transabdominal ultrasonography has shown to be an inexpensive and safe imaging method in a variety of different settings [14, 17]. Performing ultrasound evaluation before undertaking the procedure will resolve any doubt of overlying left liver lobe [14, 16]. To date, however, no data have clarifyed the role of bedside sonographic control for the positioning of enteral feeding tubes [17]. Therefore, the applications of conventional CT as a guiding tool for PRG has already been described. However, usually only gastric puncture was performed under CT control while the subsequent steps, such as guidewire and gastrostomy catheter insertion, had to be completed under conventional fluoroscopic assistance in the angiography suite [7]. Typically, the angiography suite is separated from the CT unit; therefore, it may be troublesome and time consuming to transport the patient [7, 18]. In addition, procedure times will be prolonged in this setting. CBCT is ideal when both “CT-like” and real-time fluoroscopic imaging are required for complex or high-risk interventions [19]. CBCT makes it possible to combine these modalities in the interventional radiology suite. Previously, patients were either transferred from the interventional radiology suite to the CT suite or a portable C-arm fluoroscopy unit was brought into the CT suite [19]. All of these problems can be resolved by using a C-arm angiography system equipped with CBCT technology.
The imaging information provided by CBCT allows for the acquisition and the reconstruction of CT-like images in flat-panel-based interventional suites, which allows the safest identification of an access path for tube insertion. In addition, recent advantages in overlay technology have allowed the 3D imaging information to be projected onto the fluoroscopic image (i.e., 3D roadmapping), thus enabling real-time guidance for needle or probe placement [10, 12]. Our initial experiences with this technique demonstrate the advantage of CBCT-guided PRG insertion. Although the benefits are difficult to measure, this ability does increase operator confidence and overall safety for high-risk patients [19].
In our study, puncture of the transverse colon and the left liver lobe was avoided as a result of using CBCT guidance in all cases. The guidewire can be accurately advanced to the stomach under fluoroscopy overlay guidance. The technical success rate in our study was 100% in CBCT-guided PRG. Overall, no major complications and only two minor complications occurred during the first 30 days after the procedure. The rate of complications using CBCT-guided PRG insertion in our study is comparable with the rates of adverse effects previously reported in larger series using CT guidance [7]. In addition, the rate of long-term minor complications in our series is similar to those seen in other series [20]. Furthermore, in our study, the mean procedure time for PRG insertion was 30,1 minutes compared with previously published data using CT/fluoroscopic-linked guidance (46 minutes [7]) without increasing median fluoroscopy time (4,32 minutes versus 4,59 minutes) [5].
The issue of patient dose in CBCT is complex. First, comparison with multidetector CT is complicated by lack of a universally accepted common dose metric. CT dose index (CTDI) and the dose-length product do not correctly apply to cone-beam geometrics secondary to the large z-coverage of a flat-panel detector. Second, direct comparisons in the literature are limited by lack of equivalent image quality in the resultant image sets [21]. However, it has been noted that patient dose of abdominal CBCT (CTDI 12.5 mGy for abdominal XperCT scan) is comparable with that for conventional helical CT [22]. Furthermore, CBCT has been reported to result in decreased radiation dose compared with angio-CT in head and neck applications [23]. Moreover, through 200º rotation of the gantry, the CBCT system generates tomographic data sets that have been shown experimentally to result in patient dose less than that calculated from single helical CT images [24]. The consequent acquisition of only 120 images based on 240º movement range in our examination protocol, compared with reconstructions using the same C-arm movement range but with 310 images acquired, caused additional decreases in radiation exposure to the patients in our study. Unlike multidetector CT, in which the collimation is fixed, careful consideration must be taken with CBCT to limit the imaging field of view to the anatomic area of interest. This will decrease total radiation, resulting in lower patient dose and improved image contrast through decreased scattered radiation [21].
Conclusion
In conclusion, CBCT-guided PRG is a safe and effective method for gastrostomy insertion in patients in whom endoscopic gastrostomy is not feasible. CBCT provides detailed imaging of the soft tissue and surrounding structures of the epigastric region within one diagnostic tour, which then allows the safest identification of an access path for tube insertion. Although CBCT guidance is not necessary in all cases, and the benefits are difficult to measure, this ability does increase operator confidence and overall safety for high-risk patients.
References
de Baer T, Chapot R, Kuoch V, Chevallier P, Delille JP, Domenge C et al (1999) Percutaneous gastrostomy with fluoroscopic guidance: Single-center experience in 500 consecutive cancer patients. Radiology 210:651–654
Wollman B, D’Agostino HB, Walus-Wigle JR, Easter DW, Beale A (1995) Radiologic, endoscopic, and surgical gastrostomy: An institutional evaluation and meta-analysis of the literature. Radiology 197:699–704
Laasch HU, Martin DF (2007) Radiologic gastrostomy. Endoscopy 39:247–255
Ryan JM, Hahn PF, Boland GW, McDowell RK, Saini S, Mueller PR (1997) Percutaneous gastrostomy with T-fastener gastropexy: Results of 316 consecutive procedures. Radiology 203:496–500
Thornton FJ, Fotheringham T, Haslam PJ, McGrath FP, Keeling F, Lee MJ (2002) Percutaneous radiologic gastrostomy with and without T-fastener gastropexy: A randomized comparison study. Cardiovasc Intervent Radiol 25:467–471
Preshaw RM (1981) A percutaneous method for inserting a feeding gastrostomy tube. Surg Gynecol Obstet 152:658–660
Tsukuda T, Fujita T, Ito K, Yamashita T, Matsunaga N (2006) Percutaneous radiologic gastrostomy using push-type gastrostomy tubes with CT and fluoroscopic guidance. Am J Roentgenol 186:574–576
Gottschalk A, Strotzer M, Feuerbach S, Rogler G, Seitz J, Volk M (2007) CT-guided percutaneous gastrostomy: Success rate, early and late complications. Fortschr Röntgenstr 179:387–395
Soderman M, Babic D, Holmin S, Andersson T (2008) Brain imaging with a flat detector C-arm: Technique and clinical interest of XperCT. Neuroradiology 50:863–868
Wilhlem K, Babic D (2006) 3D angiography in the interventional clinical routine. Medicamundi 50:24–31
Miyayama S, Yamashiro M, Okuda M, Yoshie Y, Sugimori N, Igarashi S et al (2009) Usefulness of cone-beam computed tomography during ultraselective transcatheter arterial chemoembolization for small hepatocellular carcinomas that cannot be demonstrated on angiography. Cardiovasc Intervent Radiol 32:255–264
Tam A, Mohamed A, Pfister M, Rohm E, Wallace MJ (2009) C-arm cone beam computed tomographic needle path overlay for fluoroscopic-guided placement of translumbar central venous catheters. Cardiovasc Intervent Radiol [Epub ahead of print]
Knackstedt C, Mülenbruch G, Mischke K, Bruners P, Schimpf T, Frechen D et al (2008) Imaging of the coronary venous system: Validation of three-dimensional rotational venous angioplasty against dual-source computed tomography. Cardiovasc Intervent Radiol 31:1150–1158
van Sonnenberg E, Wittich GR, Brown LK, Tanenbaum LB, Campbell JB, Cubberley DA et al (1986) Percutaneous gastrostomy and gastroenterostomy: 1. Techniques derived from laboratory evaluation. Am J Roentgenol 146:577–580
Silas AM, Pearce LF, Lestina LS, Grove MR, Tosteson A, Manganiello WD et al (2005) Percutaneous radiologic gastrostomy versus percutaneous endoscopic gastrostomy: A comparison of indications, complications and outcomes in 370 patients. Eur J Radiol 56:84–90
Dasari BV, Gardiner KR, Khosraviani K, Ellis P (2009) Intrahepatic delivery of feeds caused by a displaced percutaneous radiological. Br J Radiol 82:e48–e50
Gubler C, Bauerfeind P, Vavricka SR, Mullhaupt B, Fried M, Wildi SM (2006) Bedside sonographic control for positioning enteral feeding tubes: A controlled study in intensive care unit patients. Endoscopy 38:1256–1260
Rieker O, Pitton M, Herber S, Vomweg T, Teifke A, Düber C (2005) Direct percutaneous radiologic-jejunostomy (PR) and duodenostomy: A retrospective analysis. Fortschr Röntgenstr 177:393–398
Wallace MJ, Kuo MD, Glaiberman C, Binkert CA, Orth RC, Soulez G (2008) Three-dimensional C-arm cone-beam CT: Applications in the interventional suite. J Vasc Interv Radiol 19:799–813
Dinkel HP, Beer KT, Zbären P, Triller J (2002) Establishing radiological percutaneous gastrostomy with balloon-retained tubes as an alternative to endoscopic and surgical gastrostomy in patients with tumours of the head and neck or oesophagus. Br J Radiol 75:371–377
Orth RC, Wallace MJ, Kuo MD (2008) C-arm cone-beam CT: General principles and technical considerations for use in interventional radiology. J Vasc Interv Radiol 19:814–820
Racadio JM, Babic D, Homan R, Rampton JW, Patel MN, Racadio JM et al (2007) Live 3D guidance in the interventional radiology suite. Am J Roentgenol 189:W357–W364
Ishikura R, Ando K, Nagami Y, Yamamoto S, Miura K, Pande AR et al (2006) Evaluation of vascular supply with cone-beam computed tomography during intraarterial chemotherapy for a skull base tumor. Radiat Med 24:384–387
Hirota S, Nakao N, Yamamoto S, Kobayashi K, Maeda H, Ishikura R et al (2006) Cone-beam CT with flat-panel-detector digital angiography system: Early experience in abdominal interventional procedures. Cardiovasc Intervent Radiol 29:1034–1038
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Möhlenbruch, M., Nelles, M., Thomas, D. et al. Cone-Beam Computed Tomography–Guided Percutaneous Radiologic Gastrostomy. Cardiovasc Intervent Radiol 33, 315–320 (2010). https://doi.org/10.1007/s00270-009-9641-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-009-9641-4