Abstract
This study evaluated the usefulness of cone-beam computed tomography (CBCT) during ultraselective transcatheter arterial chemoembolization (TACE) for hepatocellular carcinomas (HCC) that could not be demonstrated on angiography. Twenty-eight patients with 33 angiographically occult tumors (mean diameter 1.3 ± 0.3 cm) were enrolled in the study. The ability of CBCT during arterial portography (CBCTAP), during hepatic arteriography (CBCTHA), and after iodized oil injection (LipCBCT) to detect HCC lesions was retrospectively analyzed. The technical success of TACE was divided into three grades: complete (the embolized area included the entire tumor with at least a 5-mm wide margin), adequate (the embolized area included the entire tumor but without a 5-mm wide margin in parts), and incomplete (the embolized area did not include the entire tumor) according to computed axial tomographic (CAT) images obtained 1 week after TACE. Local tumor progression was also evaluated. CBCTAP, CBCTHA, and LipCBCT detected HCC lesions in 93.9% (31 of 33), 96.7% (29 of 30), and 100% (29 of 29) of patients, respectively. A single branch was embolized in 28 tumors, and 2 branches were embolized in five tumors. Twenty-seven tumors (81.8%) were classed as complete, and 6 (18.2%) were classed as adequate. None of the tumors were classed as incomplete. Twenty-five tumors (75.8%) had not recurred during 12.0 ± 6.2 months. Eight tumors (24.2%), 5 (18.5%) of 27 complete success and 3 (50%) of 6 adequate success, recurred during 10.1 ± 6.2 months. CBCT during TACE is useful in detecting and treating small HCC lesions that cannot not be demonstrated on angiography.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carinoma (HCC) is the fifth most common cancer worldwide. Although it has become possible to diagnose small HCC lesions with advancement in imaging technology and establishment of a high-risk group, the prognosis remains poor because of the associated hepatic impairment and high intrahepatic recurrence rate [1].
Transcatheter arterial chemoembolization (TACE) is one of the effective therapeutic options for inoperable HCC lesions [1–5]. We have performed ultraselective TACE to improve local control and to decrease adverse effects [5]. However, angiography frequently cannot demonstrate small HCC lesions because of decreased hypervascularity, and TACE is reluctantly performed to a relatively large area because of difficulty in identifying the feeding vessel. Recently, cone-beam computed tomographic (CBCT) technology using a flat-panel detector (FPD) has become available [6–10], and we used this technique in combination with ultraselective TACE for HCC lesions. In this study, we analyzed the ability of several CBCT techniques to detect HCC lesions with a mean diameter of 1.3 cm as well as report the technical success rates of ultraselective TACE assisted by CBCT for HCC lesions that could not be demonstrated on angiography.
Materials and Methods
This was a retrospective study to evaluate the usefulness of CBCT during TACE procedure for small HCC lesions that could not be demonstrated on angiography. Institutional Review Board approval is not required at our institution for this type of retrospective study. Written informed consent was obtained from each patient before undergoing TACE.
Patients
Between September 2006 and March 2008, 532 TACE procedures were performed in our hospital in 299 patients with inoperable HCC lesions. Among them, we reviewed 33 angiographically occult HCC lesions of 28 patients treated with TACE assisted by CBCT technology. All tumors were diagnosed as HCC lesions based on imaging findings. The diagnosis of all HCC lesions was established by (1) a combination of nodular enhancement on arterial phase and wash of contrast material on delayed phase on double arterial-phase dynamic computed axial tomography (CAT) obtained using a multidetector-row helical CAT scanner (MDCAT; Aquilion-16 or Aquilion-64; Toshiba, Tokyo, Japan) and (2) presence of a nodular perfusion defect on CAT during arterial portography (CTAP and/or CBCTAP). In dynamic CAT, the first arterial-phase images were obtained 35 s after the start of injection of 100 ml 300 mg I/ml iohexol (Omunipaque 300; Daiichi-Sankyo, Tokyo, Japan) at a rate of 3 ml/s, and the second arterial-phase images were obtained 45 s after injection of contrast material. These two arterial-phase images were accomplished in a single breath hold. Delayed-phase images were obtained 180 s after injection of contrast material. The tumor selection criteria were as follows: (1) a tumor that did not show an obvious tumor stain on angiography and (2) a tumor that had not been previously treated, including a newly developed tumor during follow-up after HCC treatment. Thirty-three HCC lesions in 28 patients met these criteria. There were 19 men and 9 women (mean age [± SD] 71.3 ± 6.9 years [range 57 to 84]). All patients had chronic hepatitis or liver cirrhosis. This was related to hepatitis C in 19 patients, to hepatitis B in 1 patient, and to habitual alcohol complication in 2 patients. The etiology was unknown in 6 patients. Three patients had 2 tumors, including 1 patient who had 2 tumors develop asynchronously. One patient had 3 tumors. In total, these patients had 33 tumors that could not be detected on angiography (mean diameter 1.3 ± 0.3 cm [range 0.8 to 2.4 cm, median 1.2 cm]) (Table 1). Twenty-one tumors were initially demonstrated, and 12 were newly developed during follow-up after HCC treatment. Five patients also had another tumor that showed an obvious tumor stain on angiography. Serum levels of α-fetoprotein were >21 μg/l in 8 patients (21 to 100 μg/l [n = 4], 101 to 400 μg/l [n = 3], and >401 μg/l [n = 1]). Serum levels of protein induced in vitamin K absence II (PIVKA-II) were >41 mAU/ml (μg/l) in 11 patients (41 to 100 mAU/ml [n = 8], 101 to 400 mAU/ml [n = 2], and >401 mAU/ml [n = 1]). In 2 patients, serum levels of both tumor markers were elevated. In 11 patients (39.2%), serum levels of both tumor markers were normal. None of the tumors were candidates for surgical resection because of poor hepatic function reserve or patient refusal. None of the tumors were also candidates for radiofrequency (RF) ablation because of the difficulty in detecting the tumor on sonography and unenhanced CAT, presence of ascites, tumor location near vulnerable structures, or patient refusal to undergo RF ablation.
CBCT Technique
An angiographic unit with a 38 × 30 cm FPD (Allula Xper FD20; Philips Medical Systems, Best, The Netherlands) was used to obtain CBCT images. Three-hundred twelve projection images with X-ray parameters of 120 kV and 200 to 300 mAs were obtained by 10-s acquisition with 207º rotation around the patient of the FPD of the angiographic C-arm. Oxygen was administered to patients during the procedure to minimize the discomfort of breath holding.
Three different CBCT techniques (XperCT; Philips Medical Systems) were used:
-
1.
CBCTAP was routinely performed at the beginning of the procedure. Forty ml contrast material (370 mg I/ml iopamidol [Iopamiron 370; Bayer, Osaka, Japan] or 350 mg I/ml iomeprol [Iomeron 350; Ezai, Tokyo, Japan] was injected at a rate of 3 ml/s through a 4F catheter placed into the superior mesenteric artery after administration of 2.5 μg prostaglandin E1 (Liple; Mitsubishi Pharma Corporation, Osaka, Japan). When the replaced hepatic branches or the arterial flow toward the liver from the superior mesenteric artery was demonstrated, the catheter tip was deeply advanced beyond these branches. The scan began 25 s after the beginning of the injection of contrast material.
-
2.
CBCTHA was performed at the common or proper hepatic, right or left hepatic, or selected small branch of the liver. When CBCTHA was performed at the common or proper hepatic artery, 25 ml half-diluted contrast material was injected at a rate of 1.5 ml/s through a 4F catheter. When CBCTHA was performed at a more distal level of the right or left hepatic artery, 5 ml half-diluted contrast material was slowly injected manually through a 2F tip microcatheter (Progreat α; Terumo, Tokyo, Japan). The scan began 7 s after the beginning of the injection of contrast material.
-
3.
CBCT was also performed after injection of a mixture of iodized oil (Lipiodol; Andre Guerbet, Aulnaysous-Bois, France) and anticancer drugs (LipCBCT). A mixture of 1 to 3 ml iodized oil (almost equal to the diameter of the tumor, e.g., a 2-cm tumor with 2 ml iodized oil), 10 to 20 mg epirubicin (Farmorbicin; Kyowa Hakko, Tokyo, Japan), and 2 to 4 mg mitomycin C (Mitomycin; Kyowa Hakko) was prepared, and CBCT was performed after injection of approximately 1 to 2 ml of the mixture. This technique was mainly performed when blood supply to the tumor from the selected branch was highly suspected or when iodized oil accumulation in the tumor was unclear during TACE despite tumor feeding on selective CBCTHA images. In addition, this technique was performed when the selected branch was too minute and contrast material easily overflowed despite careful hand injection. When multiple small branches were each sequentially embolized after performing selective CBCTHA, we also regarded additional CBCTHA at one small branch obtained after TACE of another small branch as LipCBCT.
All CAT-like images were obtained 90 s after scanning at optional cross-section using a workstation (Philips Medical Systems). Images, 3-mm thick, were used for observation. Conventional CTAP was performed using an MDCAT scanner (Aquilion-64; Toshiba) when CBCTAP could not depict the target tumor.
TACE Procedure
We defined ultraselective TACE as TACE at the most distal portion of the subsubsegmental artery of the liver. A 2F tip microcatheter was advanced into the target branch through a 4F catheter placed in the celiac, superior mesenteric, or common hepatic artery. To navigate the microcatheter, a 0.016-inch guidewire (GT-Wire; Terumo) was routinely used in all patients. When selection of the feeding branch by the 0.016-inch guidewire was difficult, a 0.012-inch guidewire (GT-Wire; Terumo) was used. The target branch was identified according to tumor location and findings on CBCTAP and CBCTHA images.
After the microcatheter was inserted into the target branch, selective CBCTHA or LipCBCT was performed to confirm whether the branch fed the tumor. When there was no apparent feeding to the target tumor, additional CBCT was performed after another branch was selected. When blood supply to the target tumor through the selected branch was identified, TACE was performed by injection of a mixture of iodized oil and anticancer drugs followed by gelatin sponge particles (Gelfoam; Upjohn, Kalamazoo, MI [until December 2006] or Gelpart; Nihon Kayaku, Tokyo, Japan [since January 2007]). When the entire tumor was not embedded in the vascular territory of the selected branch, another feeding vessel was selected and sequentially embolized in the same fashion. The TACE procedure was ended when the entire tumor was included in the embolized area on CBCT images. In five patients who had additional tumors showing tumor stain on angiography, the tumor was sequentially treated with ultraselective TACE.
Definition of Technical Success and Follow-Up Protocol
Unenhanced CAT was performed 1 week after TACE in all patients to check iodized oil distribution not only in the tumor but also in the surrounding liver parenchyma. We defined the embolized area where iodized oil was retained at 1 week after CAT. According to these CAT images, technical success was divided into three grades: complete, adequate, and incomplete: (1) complete was defined as the embolized area included the entire tumor with at least a 5-mm wide circumferential margin; (2) adequate was defined as the embolized area included the entire tumor but the 5-mm wide safety margin was not uniformly obtained along all parts of the circumference; and (3) incomplete was defined as the embolized area did not include the entire tumor.
Dynamic CAT every 2 to 3 months after TACE procedures was also performed in all patients to check local tumor recurrence. Magnetic resonance (MR) imaging was not routinely performed and was only performed in six patients when local recurrence could not be judged on CAT because of artifact from densely accumulated iodized oil in the tumor.
Results
Results for each tumor are listed in Table 1. CBCT was performed in 2 to 6 sessions (mean 3.8 ± 1.1) in each patient. CBCTAP was performed in all patients (100%). One to five sessions (mean 2.1 ± 1.1 sessions) of CBCTHA were performed in 30 tumors (90.9%) in 26 patients. CBCTHA at the common hepatic artery (n = 10), the proper hepatic artery (n = 2), or right hepatic artery (n = 4) was performed in 16 tumors in 13 patients, and selective CBCTHA at the distal portion of the subsubsegmental artery was performed in 26 tumors in 22 patients. LipCBCT was performed in 29 tumors (87.9%) in 25 patients. In 3 of these 25 patients, LipCBCT was performed twice. Both CBCTHA and LipCBCT were performed in 25 tumors in 22 patients. Either selective CBCTHA or LipCBCT was performed in all tumors. Conventional CTAP was performed in 2 tumors (6%) in 2 patients in whom the lesions could not be detected by CBCTAP.
Ability of Each CBCT Technique to Detect HCC Lesions
All but two tumors measuring 1 cm in diameter were detected on CBCTAP (Figs. 1 and 2). Conventional CTAP depicted the remaining two tumors. Therefore, detectability of HCC lesions on CBCTAP was 93.9%.
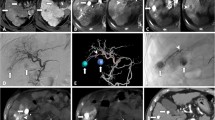
A small HCC lesion in segment VI in a 76-year-old man. A Arterial-phase CAT shows a hyperatteunating tumor in segment VI (arrow). B CBCTAP shows a tumor as a nodular perfusion defect (arrow). C Arteriogram of the common hepatic artery does not show any obvious tumor stains. D CBCTHA at the common hepatic artery shows nodular enhancement (arrow). E Arteriogram of the posterior inferior subsegmental artery (A6) also does not show any tumor stains. The small branch derived from the proximate portion of A6 was suspected of being the tumor-feeding branch and was selected for embolization (arrow). F Spot radiograph obtained during iodized oil injection through the branch does not show obvious iodized oil accumulation in the tumor. G LipCBCT obtained after iodized oil injection clearly shows that the entire tumor is embedded in the territory of the embolized area. TACE was performed after additional injection of iodized oil. H CAT obtained 1 week after TACE shows dense iodized accumulation throughout the entire tumor, including a safety margin. I Arterial-phase CAT obtained 10 months after TACE shows dense iodized oil accumulation in the tumor without recurrence
A small HCC lesion at the boundary between segments VII and VIII in a 71-year-old man. A CBCTAP shows a nodular perfusion defect at the boundary between segments VII and VIII (arrow). B Arteriogram of the right hepatic artery does not show any obvious tumor stains. C Selective arteriogram of the branch of the posterior superior subsegmenal artery does not show any tumor stains. D Selective CBCTHA at the branch showed that the branch partially feeds the tumor (arrow). TACE of this branch was performed. E Selective arteriogram of the branch of the anterior superior subsegmental artery shows no tumor stains. Selective CBCTHA at the branch shows that this branch also feeds the tumor (not shown). TACE of the branch was started at this point. F LipCBCT at the branch shows dense iodized oil accumulation throughout the entire tumor (arrow). G Arterial-phase CAT obtained 14 months after TACE shows that the tumor has decreased in size without any evidence of local recurrence (arrow). Atrophy of the surrounding liver parenchyma is also seen
CBCTHA demonstrated 29 (96.7%) of 30 tumors (Figs. 1 through 3). The remaining tumor (1-cm diameter) that was not detected on CBCTAP was also undetectable on CBCTHA performed at the common hepatic artery. LipCBCT clearly depicted this lesion. Another tumor (1-cm diameter) that could not be detected on CBCTAP was detected on CBCTHA.
A small tumor in segment VII in a 74-year-old woman. A Arterial-phase CAT shows a hyperatteunating tumor in segment VII (arrow). B Celiac arteriogram does not show any tumor stains. C Selective arteriogram of the branch of the posterior superior subsegmental artery also does not show any obvious tumor stains. D CBCTHA at the branch shows enhancing tumor (arrow). E LipCBCT shows a dense iodized oil accumulation in the tumor more clearly than CBCTHA images. F Arterial-phase CAT 12 months after TACE shows that the tumor has decreased in size without any evidence of local recurrence (arrow). Atrophy of the surrounding liver parenchyma is also seen
LipCBCT demonstrated 29 (100%) of 29 tumors (Figs. 1 through 3). In 25 tumors that underwent both selective CBCTHA and LipCBCT, all tumors were depicted more clearly by LipCBCT than by selective CBCTHA (Fig. 3), although artifacts from densely accumulated iodized oil were observed in several tumors.
Technical Success Rates and Local Effects of Ultraselective TACE
Ultraselective TACE was performed in all 33 tumors. A single branch was embolized in 28 tumors (Figs. 1 and 3) (84.6%), and 2 branches were embolized in 5 tumors (15.4%) after confirmation of additional supply from another branch by selective CBCTHA and/or LipCBCT (Fig. 2).
Complete success was achieved in 27 tumors (81.8%) (Figs. 1 through 3), and adequate success was achieved in 6 tumors (18.2%). No tumors demonstrated incomplete embolization. All 33 tumors were followed-up during the mean follow-up period of 13.1 ± 6.0 months. Twenty-five tumors (75.8%) had not recurred during 4 to 25 months (mean 12.0 ± 6.2) after the procedure (Figs. 1 through 3). In the remaining 8 tumors (24.2%), 5 (18.5%) of 27 had complete success, and 3 (50%) of 6 had adequate success; local recurrence was observed from 2 to 20 months (mean 10.1 ± 6.2) after TACE. Additional TACE was performed in 5 tumors in 5 patients, and the recurrent tumor was supplied by another feeding artery. The remaining 3 tumors were followed-up because the recurrent tumors were small. Two patients died during follow-up. One patient died from rapid tumor progression at other sites 16 months after TACE, and another died from arrhythmia 8 months after TACE without tumor recurrence.
Discussion
TACE is one of the effective therapeutic options for inoperable HCC lesions, and it is widely performed throughout the world [1–5]. With advancement of imaging modalities, such as the MDCAT scanner, smaller HCC lesions are being detected [11]. Despite advances in the angiography unit, these tumors frequently cannot be demonstrated by angiography because the lesions are small and have a less hypervascular nature. A combined CAT-angiography system (Interventional radiology-CAT) is useful to detect these small lesions; however, the system is expensive and requires a large room [12, 13].
CBCT is a new technology to obtain CAT-like images using a C-arm system that rotates around the patient [14, 15]. A C-arm system equipped with a large FPD can obtain high image quality and large FOV compared with that obtained by the image-intensified system. Recently, this technology has been introduced into several interventional procedures [6–10]. CAT-like images can be easily obtained during the procedure simply by lifting the patients’ hands without causing other movement.
We combined CBCT technology with TACE for HCC lesions that could not be demonstrated on angiography. CBCT demonstrated sufficient ability to detect small HCC lesions, although these images were slightly noisy. In the present study, the ability of CBCTAP to detect HCC lesions was 93.9%, and two tumors measuring 1 cm in diameter could not be detected. In our previous report [9], CBCTAP depicted approximately 89% of HCC nodules, including eight suboptimal lesions, compared with conventional CTAP. CBCTHA and LipCBCT also showed sufficient detection ability, being able to detect 96.7% and 100% of tumors, respectively. In addition, selective CBCTHA and LipCBCT at the TACE point were also useful for not only depicting the tumor but also for monitoring the embolized area. Observations on optional cross-sections can easily provide information on whether the embolized area includes the entire target tumor with an appropriate safety margin. LipCBCT can depict the tumor as a hyperattenuating lesion more clearly compared with CBCTHA because of the high contrast of iodized oil, although artifacts from densely accumulated iodized oil infrequently may decrease the image quality.
Histopathologically, even small HCC lesions have microsatellite lesions. Sasaki et al [16]. reported that microsatellites were observed in 7 (29.2%) of 24 tumors <25 mm in diameter, and all but 1 microsatellite were located within 5 mm from the main tumor. Therefore, we supposed that a safety margin of the embolized area around a small tumor was at least 5-mm wide. In contrast, the embolized area should be minimized considering the risk of adverse effects. This is the dilemma of ultraselective TACE. The smaller the area embolized, the more likely TACE will be insufficient without an adequate safety margin. Especially in a tumor located at the boundary between different small branches, a safety margin around the tumor frequently may not be obtained. Therefore, intraprocedural monitoring of the embolized area is necessary to achieve the complete success of TACE. In the present study, however, a safety margin around the entire tumor was not obtained in 18.2% of tumors. This limitation of TACE therapy is dependent on the vascular territory of the selected branch. Peritumoral recurrence may occur during long-term follow-up in such tumors without a safety margin. In fact, 50% of tumors with insufficient safety margins recurred during follow-up in the present study.
There are several limitations to the present study. First, all tumors were diagnosed based on imaging findings without being histologically proven. In addition, serum levels of tumor markers were not elevated in 39.2% of patients because of small tumor size and/or early-stage HCC. However, we consider that advances in imaging modalities can facilitate establishment of an HCC diagnosis without biopsy. Second, the “gold standard” for diagnosis of HCC in this study was dynamic CAT and CBCTAP or conventional CTAP findings. This means that any HCC nodules that were not detected by these CAT or CAT-like images were not evaluated in the present study. However, we consider that a combination of dynamic CAT obtained by MDCAT scanner and CBCTAP or conventional CTAP has sufficient ability to detect small HCC lesions that require treatment [9, 11]. Third, the present study mainly evaluated the technical aspects of TACE for HCC lesions that were undetectable on angiography, and follow-up has not yet been sufficient to conclude the efficacy of ultraselective TACE for such small tumors. Fourth, the indications and timing of TACE for such small tumors may be controversial. However, adverse effects, such as impairment of hepatic function reserve and hepatic arterial damage, can be minimized if complete treatment is performed in a small focus. In addition, ultraselective TACE has stronger therapeutic effects on early-stage HCC lesions compared with those of conventional TACE [17]. We consider that ultraselective TACE in a highly limited area and assisted by CBCT technology for small tumors may improve the prognosis of patients with HCC. Finally, local tumor recurrence was judged by CAT alone in almost all patients. Hyperattenuating iodized oil in the tumor impairs assessment of residual tumor enhancement on contrast-enhanced CAT [18]. In a report by Kim et al. [19], MR had higher sensitivity for small HCC lesions (≤1 cm) compared with MDCAT. In addition, Kamel et al. [20] reported that the amount of iodized oil deposition alone could not be used as a consistent predictor of tumor response and that dynamic contrast-enhanced and diffusion-weighted MR imaging could potentially provide effective markers of treatment response and tumor cell death after TACE. Periodic MR imaging should be necessary to improve tumor response interpretation.
CBCT technology is also useful in all TACE procedures for HCC lesions, although we only evaluated its usefulness for small HCC lesions that could not be demonstrated by angiography in the present study. As indicated previously, intraprocedual monitoring of the embolized area by CBCT may improve the success rates and therapeutic effects of TACE, and we have routinely performed CBCT during approximately 760 TACE procedures for HCC lesions since May 2006. In the future, selective CBCTHA may be performed instead of selective angiography at the minute feeding branch of small tumors because the information about whether the entire tumor is included in the vascular territory of the selected branch is more important than whether the selected branch is fed by the tumor. Two-dimensional angiography cannot provide this information in small tumors. Further technical advances, such as improvement of image quality (improvement of soft tissue contrast and reduction of artifacts), shortening of acquisition and reconstruction times, and advances in the image display console may be needed to more easily perform several CBCT sessions during a single TACE procedure. In addition, the patients’ hands are unstable during CBCT acquisition. We now employ a hand-fashioned grip above the patients’ heads to keep their hands lifted during the procedure.
In conclusion, CBCT technology during TACE was useful in detecting and treating small HCC lesions that could not be demonstrated on angiography. This technology may make possible ultraselective TACE for these HCC lesions. In addition, it may improve the success rates and therapeutic effects of ultraselective TACE for small HCC lesions.
References
Takayasu K, Arii S, Ikai I et al (2006) Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology 131:461–469
Matsui O, Kadoya M, Yoshikawa J et al (1993) Small hepatocellular carcinoma: treatment with subsegmental transcatheter arterial embolization. Radiology 188:79–83
Yamada R, Sato M, Kawabata M et al (1983) Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology 148:397–401
Uchida H, Ohishi H, Matsuo N et al (1990) Transcatheter hepatic segmental arterial embolization using Lipiodol mixed with an anticancer drug and Gelfoam particles for hepatocellular carcinoma. Cardiovasc Intervent Radiol 13:140–145
Miyayama S, Matsui O, Yamashiro M et al (2007) Ultraselective transcatheter arterial chemoembolization with a 2-F tip microcatheter for small hepatocellular carcinomas: relationship between local tumor recurrence and visualization of the portal vein with iodized oil. J Vasc Interv Radiol 18:365–376
Binkert CA, Alencar H, Singh J et al (2006) Translumbar type II endoleak repair using angiographic CT. J Vasc Interv Radiol 17:1349–1353
Hirota S, Nakao N, Yamamoto S et al (2006) Cone-beam CT with flat-panel-detector digital angiography system: early experience in abdominal interventional procedures. Cardiovasc Intervent Radiol 29:1034–1038
Georgiades CS, Hong K, Geschwind JF et al (2007) Adjunctive use of C-arm CT may eliminate technical failure in adrenal vein sampling. J Vasc Interv Radiol 18:1102–1105
Miyayama S, Matsui O, Yamashiro M et al (2008) Detection of hepatocellular carcinoma by CT during arterial portography using a cone-beam CT technology: comparison with conventional CTAP. Abdom Imaging (in press)
Virmani S, Ryu RK, Sato KT et al (2007) Effect of C-arm angiographic CT on transcatheter arterial chemoembolization of liver tumors. J Vasc Interv Radiol 18:1305–1309
Murakami T, Kim T, Takamura M et al (2001) Hypervascular hepatocellular carcinoma: detection with double arterial phase multi-detector row helical CT. Radiology 218:763–767
Ishijima H, Koyama Y, Aoki J et al (1999) Use of a combined CT-angiography system for demonstration of correlative anatomy during embolotherapy for hepatocellular carcinoma. J Vasc Interv Radiol 10:811–815
Takayasu K, Muramatsu Y, Maeda T et al (2001) Targeted transarterial oily chemoembolization for small foci of hepatocellular carcinoma using a unified helical CT and angiography system: analysis of factors affecting local recurrence and survival rates. AJR Am J Roentgenol 176:681–688
El-Sheik M, Heverhagen JT, Alfke H et al (2001) Multiplanar reconstructions and three-dimensional imaging (computed rotational osteography) of complex fractures by using a C-arm system: initial results. Radiology 221:843–849
Linsenmaier U, Rock C, Euler E et al (2002) Three-dimensional CT with a modified C-arm image intensifier: feasibility. Radiology 224:286–292
Sasaki A, Kai S, Iwashita Y et al (2005) Microsatellite distribution and indication for locoregional therapy in small hepatocellular carcinoma. Cancer 103:299–306
Miyayama S, Matsui O, Yamashiro M et al (2007) Iodized oil accumulation in the hypovascular tumor portion of early-stage hepatocellular carcinoma after ultraselective transcatheter arterial chemoembolization. Hepatol Int 1:451–459
Kamel IR, Bluemke DA, Ramsey D et al (2003) Role of diffusion-weighted imaging in estimating tumor necrosis after chemoembolization of hepatocellular carcinoma. AJR Am J Roentgenol 181:708–710
Kim YK, Kim CS, Chung GH et al (2006) Comparison of gadobenate dimeglumine-enhanced dynamic MRI and 16-MDCT for the detection of hepatocellular carcinoma. AJR Am J Roentogenol 186:149–157
Kamel IR, Bluemke DA, Eng J et al (2006) The role of functional MR imaging in the assessment of tumor response after chemoembolization in patients with hepatocellular carcinoma. J Vasc Interv Radiol 17:505–512
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miyayama, S., Yamashiro, M., Okuda, M. et al. Usefulness of Cone-Beam Computed Tomography During Ultraselective Transcatheter Arterial Chemoembolization for Small Hepatocellular Carcinomas that Cannot be Demonstrated on Angiography. Cardiovasc Intervent Radiol 32, 255–264 (2009). https://doi.org/10.1007/s00270-008-9468-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-008-9468-4