Abstract
Acute pneumothorax is a frequent complication after percutaneous pulmonary radiofrequency (RF) ablation. In this study we present three cases showing delayed development of pneumothorax after pulmonary RF ablation in 34 patients. Our purpose is to draw attention to this delayed complication and to propose a possible approach to avoid this major complication. These three cases occurred subsequent to 44 CT-guided pulmonary RF ablation procedures (6.8%) using either internally cooled or multitined expandable RF electrodes. In two patients, the pneumothorax, being initially absent at the end of the intervention, developed without symptoms. One of these patients required chest drain placement 32 h after RF ablation, and in the second patient therapy remained conservative. In the third patient, a slight pneumothorax at the end of the intervention gradually increased and led into tension pneumothorax 5 days after ablation procedure. Underlying bronchopleural fistula along the coagulated former electrode track was diagnosed in two patients. In conclusion, delayed development of pneumothorax after pulmonary RF ablation can occur and is probably due to underlying bronchopleural fistula, potentially leading to tension pneumothorax. Patients and interventionalists should be prepared for delayed onset of this complication, and extensive track ablation following pulmonary RF ablation should be avoided.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Percutaneous thermal ablation is a minimally invasive therapy for the treatment of hepatic and nonhepatic tumors. Radiofrequency (RF) ablation gained importance in the therapy of primary and secondary liver tumors and is an established treatment of osteoid osteoma [1–4]. Renal cell carcinoma and pulmonary malignancies represent possible new indications for image-guided RF ablation [5–7]. Evaluation of pulmonary RF ablation exhibits particular relevance due to the high incidence of primary and secondary pulmonary malignancies [7, 8]. Currently the safety and efficacy of pulmonary RF ablation are under evaluation [9–11].
Image-guided RF ablation in liver tissue is a well-tolerated technique with a relatively low rate of complications [12]. RF ablation of lung tissue is characterized by the possibility of specific complications. The most common complications after pulmonary RF ablation are self-limiting pleural effusion and pneumothorax [11, 13]. The frequency of pneumothorax after pulmonary RF ablation ranges between 9% and 67% [9, 10], whereas the rate of clinically significant pneumothoraces that require chest tube placement is reported to be about 10–20% [9, 11].
In this report we present a subset of 3 cases from a total number of 34 patients treated by 44 pulmonary RF ablation procedures. These cases showed delayed development of pneumothorax after RF ablation, and our purpose is to draw attention to the possible delayed onset of this complication.
Materials and Methods
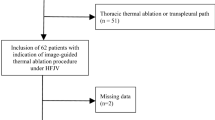
Percutaneous RF ablations were performed at two radiological centers. The treatment was approved by each Institutional Review Board. Indication for CT-guided RF ablations was based on an interdisciplinary decision and written informed consent was obtained from all patients before RF ablation. A total number of 44 RF ablation procedures were performed in 34 patients for the treatment of 47 pulmonary malignancies. The patient characteristics are summarized in Table 1. In three cases, delayed development of pneumothorax occurred. We defined delayed development of pneumothorax as delayed onset of a clinically significant pneumothorax representing a major complication according to the Society of Interventional Radiology (SIR) classification [14]. The pneumothorax demonstrates delayed progression after initially being absent at the end of the ablation procedure or being present in the form of a small pneumothorax at the end of the intervention.
Case Reports
Case 1
A 48-year-old woman underwent RF ablation for the treatment of a single pulmonary metastasis (2.1 cm) in the lower lobe of the left lung. The primary tumor was a low-grade fibromyxoid sarcoma located in the left lower leg. The lung nodule increased in size on follow-up CT and was, therefore, considered to be a lung metastasis. The patient participated in a study comprising pulmonary RF ablation followed by surgical resection 3 days later. The ablation procedure was performed under general anesthesia. A monopolar internally cooled single RF electrode with a noninsulated tip of 3 cm (Cool-tip; Valleylab, Boulder, CO, USA) was placed under CT fluoroscopy. After verification of the electrode position (Fig. 1A) RF energy was delivered using an impedance-controlled RF generator (CC; Valleylab), operating at a frequency of 480 kHz and providing a maximum power output of 200 W. The RF electrode was repositioned once, and the total duration of energy application was 23 min. The temperature at the electrode tip was 75°C measured immediately after the first application of RF energy and 68°C after the second application of RF energy. At the first electrode position, the mean power output was 101 W and the mean impedance was 116 Ω. At the second electrode position, the mean power output was 100 W and the mean impedance was 103 Ω. Afterward, the RF electrode was removed without internal cooling for coagulation of the needle track. Subsequently, postinterventional CT imaging was performed (Fig. 1B). After the ablation procedure, the patient was transferred to the Department of Thoracic, Cardiac and Vascular Surgery. Three days after pulmonary RF ablation, surgical resection was performed.
Radiofrequency (RF) ablation of a pulmonary metastasis from low-grade fibromyxoid sarcoma. A Verification of a correct applicator position by multinplanar CT imaging angulated on the shaft of the RF electrode. B Chest X-ray 4 h after the ablation procedure reveals a small pneumothorax (arrows), with a distance of 0.5 cm between visceral and parietal pleura. C Chest X-ray 32 h after the ablation procedure shows a progressive pneumothorax (arrows) with a distance of 5 cm between visceral and parietal pleura. D Photograph of the ablation zone during surgical resection. The hemorrhagic rim surrounding the ablation zone in the lower lobe can be delineated (white arrow). Furthermore, the ablation zone traverses the oblique fissure of the left lung and extends into the upper lobe (white arrow head). The former entry point (black arrow) of the RF electrode at the visceral pleura is still visible and surrounded by a hemorrhagic rim (black arrow head). Bronchopleural fistula was detected in this location
Case 2
A 68-year-old man underwent CT-guided RF ablation of a single pulmonary metastasis (0.9 cm) in the upper lobe of the right lung. The primary tumor was a malignant melanoma. The ablation procedure was performed under local anesthesia, in combination with conscious sedation. A monopolar RF system (RF 3000; Boston Scientific, Natick, MA, USA) was used, operating at a frequency of 480 kHz and providing a maximum power output of 200 W. After placement of a multitined expandable RF electrode (LeVeen; Boston Scientific), the applicator was deployed to a diameter of 3 cm and RF energy was applied. The RF electrode was repositioned once, and the total duration of energy application was 27 min. At the first electrode position, the maximum power output was 160 W. At the second electrode position, the maximum power output was 90 W. Afterward, the RF electrode was removed and a track ablation was performed along the access path throughout the pleura. Subsequent to postinterventional CT imaging, the patient was transferred to a ward.
Case 3
A 61-year-old man with synchronous liver and lung metastases from colorectal cancer underwent RF ablation of a single pulmonary metastasis in the upper lobe of the right lung. Follow-up indicated complete coagulation of this metastasis, and a new lung metastasis was detected 12 months after ablation therapy in the lower lobe of the right lung; thus, another RF ablation of the new metastasis (1.4 cm) was considered. The ablation procedure was performed under local anesthesia in combination with conscious sedation. A monopolar RF system (Model 1500; RITA, Mountain View, CA, USA) was used, operating at a frequency of 460 kHz and providing a maximum power output of 150 W. The noninsulated tips of the RF electrode (Starburst; RITA) were stepwise deployed after correct applicator placement (Fig. 2A). RF energy was applied at a total number of five positions over a total duration of 17 min, whereas the duration of energy application including the heating phases was 36 min. The temperature at the electrode tip, measured immediately after application of RF energy, was 75°C. The mean power output was 105 W and the mean impedance was 70 Ω. Afterward, the RF electrode was removed and a track ablation was performed along the access path throughout the pleura. Subsequent to postinterventional CT imaging, the patient was transferred to a ward.
Radiofrequency (RF) ablation of a colorectal lung metastasis. A CT imaging during placement of the RF electrode in a pulmonary metastasis in the lower lobe of the right lung. B Chest X-ray 3 days after the ablation procedure indicates a pneumothorax (arrows), with a distance of 0.4 cm between visceral and parietal pleura. C Chest X-ray 5 days after RF ablation shows a tension pneumothorax (arrows). D CT imaging during CT-guided placement of a chest drain shows tension pneumothorax and pleural effusion. Air formation along the former needle track (arrow) was considered to be an underlying bronchopleural fistula due to tissue disruption
Results
In our series, a total number of 11 pneumothoraces occurred after 44 interventions (25.0%) in 34 patients. Chest drain placement was necessary after 5 of 44 interventions (11.4%). Bronchopleural fistula was diagnosed subsequent to 2 of 44 interventions (4.5%). Delayed development of pneumothorax occurred subsequent to 3 of 44 interventions (6.8%).
Case 1
RF ablation was technically successful. The metastasis was encompassed by thermally induced ground-glass opacities. No complications were encountered during the ablation procedure. In particular, there was no pneumothorax seen on postinterventional CT imaging.
Postinterventional observation was uneventful except for mild pain. Vital signs were normal and blood tests were performed to exclude asymptomatic hemorrhage. Chest X-ray 4 h after the ablation procedure revealed a small apical pneumothorax (Fig. 1B). The initial therapy remained conservative. The patient did not complain of dyspnea during further observation. Chest X-ray 32 h after the ablation procedure revealed pneumothorax, with a maximum distance of 5 cm between visceral and parietal pleura (Fig. 1C). Subsequently, chest tube placement was performed at the Department of Thoracic, Cardiac and Vascular Surgery, leading to re-expansion of the left lung.
Three days after pulmonary RF ablation, surgical resection was performed. The ablation zone in the lower lobe of the left lung could be easily delineated due to the hemorrhagic rim at the periphery. Moreover, the ablation zone traversed the oblique fissure of the left lung and incorporated the dorsal part of the upper lobe. Bronchopleural fistula was detected along the former electrode track. The entry point of the RF electrode at the visceral pleura was still visible and surrounded by a hemorrhagic rim due to coagulation of the needle track (Fig. 1D). The patient remained tumor-free during the 14 months of follow-up.
Case 2
RF ablation was technically successful. The metastasis was encompassed by thermally induced ground-glass opacities. No complications were encountered during the ablation procedure. In particular, there was no pneumothorax seen on postinterventional CT imaging.
Postinterventional observation was uneventful and vital signs were normal. Chest X-ray 4 h after ablation procedure revealed an asymptomatic apical pneumothorax, with a distance of 2.4 cm between the visceral and the parietal pleura. Due to the pneumothorax prolonged hospitalization was considered to be required. Follow-up chest X-ray showed a slowly resolving pneumothorax during a period of 5 days, and the therapy remained conservative. The patient remained tumor-free during the 4 months of follow-up.
Case 3
RF ablation was technically successful. The metastasis was encompassed by thermally induced ground-glass opacities. No complications were encountered during the ablation procedure. Postinterventional CT imaging showed a small pneumothorax, with a maximum size of 1.9 cm, along the former track of the RF electrode. Since the patient was clinically stable and showed no clinically relevant symptoms, the initial therapy remained conservative. During postinterventional observation, successive dyspnea developed and chest X-ray showed a small pneumothorax of the right lung (Fig. 2B). Three days after ablation therapy, clinical symptoms were added by coughing and chest X-ray showed a slightly increasing pneumothorax. The therapy for pneumothorax remained conservative. Five days after RF ablation, dyspnea exacerbated and tension pneumothorax was confirmed by chest X-ray (Fig. 2C). Subsequently, a chest tube was inserted under CT guidance to evacuate the pneumothorax. In addition, a purulent pleural effusion was aspirated. CT imaging showed air formation along the former needle-track (2 × 0.3 cm), which was considered to be an underlying bronchopleural fistula (Fig. 2D). Chest X-ray showed re-expansion of the right lung. Chest drain and antibiotic medication led to a complete recovery. The patient was discharged from the hospital 16 days after the ablation procedure. The patient remained tumor-free during the 4 months of follow-up.
Discussion
Pneumothorax is a frequent complication after percutaneous pulmonary interventions. In a meta-analysis by Lacasse et al., the incidence of pneumothorax after transthoracic needle aspiration biopsy in 36 studies ranged from 3.1% to 41.7% (pooled incidence rate, 24.5%), whereas the incidence of pneumothorax requiring chest tube drainage was reported in 39 studies and ranged from 0% to 16.6% (pooled incidence rate, 6.8%) [15]. The frequency of pneumothorax after pulmonary RF ablation ranges between 9% and 67% [9, 10]. In a study by Hiraki et al., the incidence of pneumothorax was 52% after 224 pulmonary RF ablation procedures, and frequency of chest tube placement was 21% [11]. In a study by Okuma et al. the rate of pneumothorax was 30% after 112 RF ablation procedures, whereas chest tube placement was required in 2% [16]. In a study by Yamagami et al. the rate of pneumothorax was 30% after 129 RF ablation procedures, whereas in 11% manual aspiration of pneumothorax was required and chest tube placement was required in 4% [17]. In a study by Sano et al., pneumothorax occurred in 52% of 211 ablation procedures, requiring aspiration in 9% and chest tube placement in 12% [18]. In a study by de Baere et al. the frequency of pneumothorax was 54% after 74 sessions of pulmonary RF ablation, in 23% the pneumothorax was aspirated, and chest tube placement was performed in 9% [19]. In a study by Gillams and Lees in 37 patients, 21 pneumothoraces occurred after the treatment of 79 tumors in 55 lungs, whereas the number of tumors, number of electrode positions, and length of trajectory through aerated lung tissue impacted on the likelihood of pneumothorax [20].
The delayed development of pneumothorax after percutaneous pulmonary interventions may occur [21, 22]. In a study by Perlmutt et al., 673 transthoracic needle-aspiration procedures were performed. Pneumothorax occurred in 23.8%, and 11.5% of these required a chest tube or aspiration. In 89%, pneumothoraces were detected immediately, 9% were first seen after 1 h, and 2% were first seen after 4 h [23]. Of the pneumothoraces that required intervention, 88% were detected immediately, while the remainder were first detected after 1 h [23]. In a study by Choi et al. the incidence rate of pneumothorax was 21.8% after transthoracic needle biopsy in 458 patients, and the rate of delayed pneumothorax more than 3 h after biopsy was 3.3% [22]. However, there are only a few reports about delayed onset of pneumothorax after transthoracic needle biopsy [21, 22] or pulmonary RF ablation [6, 17]. In the report by Dupuy et al., a patient had no pneumothorax on the initial chest radiographs, while chest CT, 2 days after RF ablation for evaluation of chest pain, showed a small pneumothorax that did not warrant intervention [6].
In our series, three cases showed delayed development of pneumothorax after pulmonary RF ablation. Underlying bronchopleural fistula along the coagulated former electrode track was diagnosed in two patients. This complication may occur asymptomatically, as shown in the first and second cases, but may also lead to tension pneumothorax that requires immediate therapy, as shown in the third case. In one patient, underlying bronchopleural fistula was confirmed by subsequent surgery. We hypothesize that coagulation of the needle track may have contributed to an air leakage at the former entry point of the RF electrode. In the first patient, pathology confirmed that the former entry point of the RF electrode was not sealed 3 days after the ablation procedure. Coagulation of the visceral pleura and adjacent lung tissue leads to dehydration and may reduce the elastic properties of the lung tissue to close up the puncture. In addition, an active repair process to seal the puncture of the RF electrode may be impaired in the nonvital tissue surrounding the entry point and needle track. Moreover, rupture of lung tissue may occur in this localization. Bronchopleural fistula after pulmonary RF ablation may be difficult to treat [19, 24]. Therefore, the possibility to induce a tissue disruption or bronchopleural fistula along the needle track may be reduced if an extensive track ablation is avoided. On the other hand, as shown in hepatic RF ablation, coagulation of the needle track can prevent bleeding and tumor seeding [25]. Therefore, track ablation is an accepted technique in hepatic RF ablation [25]. In contrast, coagulation of the needle track is inconsistently applied in pulmonary RF ablation [9, 26]. As already reported by Yamakado et al. lack of track ablation can cause tumor seeding in pulmonary RF ablation [27]. Partial ablation of the electrode track may offer a reasonable compromise between the possible advantages and disadvantages of track ablation; i.e., we suggest coagulation of the electrode track when the RF electrode is removed or repositioned, except for the period when the active tip of the RF electrode passes the visceral pleura. This may prevent the risk of bleeding and tumor seeding; moreover, this may not affect the sealing of the visceral pleura. The prerequisite for this technique is an adequate margin of normal lung tissue between the entry point at the visceral pleura and the tumor tissue. Another strategy to reduce the risk of tumor cell seeding may be the use of a coaxial needle device for RF ablation. In addition, application of an autologous blood clot may improve the sealing of the needle track, as shown in lung biopsies [28]. A concluding recommendation regarding track ablation or alternative strategies in pulmonary RF ablation has to be based on further experience and evaluation of pulmonary RF ablation.
In conclusion, delayed pneumothorax development after pulmonary RF ablation can occur and may lead to tension pneumothorax. Track ablation may contribute to underlying bronchopleural fistula. Thus, extensive track ablation along the access path, subsequent to pulmonary RF ablation, should be avoided. Patients and interventionalists should be aware of the possible delayed onset of this complication. Patients should be informed about a potential occurrence after discharge from the hospital, and be advised to seek medical help if they develop chest pain or dyspnea.
References
Friedman M, Mikityansky I, Kam A et al (2004) Radiofrequency ablation of cancer. Cardiovasc Interv Radiol 27(5):427–434
Choi D, Lim HK, Rhim H et al (2007) Percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma as a first-line treatment: long-term results and prognostic factors in a large single-institution series. Eur Radiol 17(3):684–692
Veltri A, Sacchetto P, Tosetti I et al (2008) Radiofrequency ablation of colorectal liver metastases: small size favorably predicts technique effectiveness and survival. Cardiovasc Interv Radiol 31(5):948–956
Rosenthal DI, Hornicek FJ, Torriani M et al (2003) Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology 229(1):171–175
Breen DJ, Rutherford EE, Stedman B et al (2007) Management of renal tumors by image-guided radiofrequency ablation: experience in 105 tumors. Cardiovasc Interv Radiol 30(5):936–942
Dupuy DE, Zagoria RJ, Akerley W et al (2000) Percutaneous radiofrequency ablation of malignancies in the lung. AJR 174(1):57–59
Lencioni R, Crocetti L, Cioni R et al (2004) Radiofrequency ablation of lung malignancies: where do we stand? Cardiovasc Interv Radiol 27(6):581–590
Jemal A, Siegel R, Ward E et al (2008) Cancer statistics, 2008. CA Cancer J Clins 58(2):71–96
Suh RD, Wallace AB, Sheehan RE et al (2003) Unresectable pulmonary malignancies: CT-guided percutaneous radiofrequency ablation—preliminary results. Radiology 229(3):821–829
Belfiore G, Moggio G, Tedeschi E et al (2004) CT-guided radiofrequency ablation: a potential complementary therapy for patients with unresectable primary lung cancer—a preliminary report of 33 patients. AJR 183(4):1003–1011
Hiraki T, Tajiri N, Mimura H et al (2006) Pneumothorax, pleural effusion, and chest tube placement after radiofrequency ablation of lung tumors: incidence and risk factors. Radiology 241(1):275–283
Livraghi T, Solbiati L, Meloni MF et al (2003) Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology 226(2):441–451
Jin GY, Lee JM, Lee YC et al (2004) Primary and secondary lung malignancies treated with percutaneous radiofrequency ablation: evaluation with follow-up helical CT. AJR 183(4):1013–1020
Sacks D, McClenny TE, Cardella JF et al (2003) Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol 14(9; Pt 2):S199–S202
Lacasse Y, Wong E, Guyatt GH et al (1999) Transthoracic needle aspiration biopsy for the diagnosis of localised pulmonary lesions: a meta-analysis. Thorax 54(10):884–893
Okuma T, Matsuoka T, Yamamoto A et al (2008) Frequency and risk factors of various complications after computed tomography-guided radiofrequency ablation of lung tumors. Cardiovasc Interv Radiol 31(1):122–130
Yamagami T, Kato T, Hirota T et al (2006) Pneumothorax as a complication of percutaneous radiofrequency ablation for lung neoplasms. J Vasc Interv Radiol 17(10):1625–1629
Sano Y, Kanazawa S, Gobara H et al (2007) Feasibility of percutaneous radiofrequency ablation for intrathoracic malignancies: a large single-center experience. Cancer 109(7):1397–1405
de Baere T, Palussiere J, Auperin A et al (2006) Midterm local efficacy and survival after radiofrequency ablation of lung tumors with minimum follow-up of 1 year: prospective evaluation. Radiology 240(2):587–599
Gillams AR, Lees WR (2007) Analysis of the factors associated with radiofrequency ablation-induced pneumothorax. Clin Radiol 62(7):639–644
Traill ZC, Gleeson FV (1997) Delayed pneumothorax after CT-guided percutaneous fine needle aspiration lung biopsy. Thorax 52(5):581–582
Choi CM, Um SW, Yoo CG et al (2004) Incidence and risk factors of delayed pneumothorax after transthoracic needle biopsy of the lung. Chest 126(5):1516–1521
Perlmutt LM, Braun SD, Newman GE et al (1986) Timing of chest film follow-up after transthoracic needle aspiration. AJR 146(5):1049–1050
Sakurai J, Hiraki T, Mukai T et al (2007) Intractable pneumothorax due to bronchopleural fistula after radiofrequency ablation of lung tumors. J Vasc Interv Radiol 18(1):141–145
Mulier S, Mulier P, Ni Y et al (2002) Complications of radiofrequency coagulation of liver tumours. Br J Surg 89(10):1206–1222
Lee JM, Jin GY, Goldberg SN et al (2004) Percutaneous radiofrequency ablation for inoperable non-small cell lung cancer and metastases: preliminary report. Radiology 230(1):125–134
Yamakado K, Akeboshi M, Nakatsuka A et al (2005) Tumor seeding following lung radiofrequency ablation: a case report. Cardiovasc Interv Radiol 28(4):530–532
Lang EK, Ghavami R, Schreiner VC et al (2000) Autologous blood clot seal to prevent pneumothorax at CT-guided lung biopsy. Radiology 216(1):93–96
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Clasen, S., Kettenbach, J., Kosan, B. et al. Delayed Development of Pneumothorax After Pulmonary Radiofrequency Ablation. Cardiovasc Intervent Radiol 32, 484–490 (2009). https://doi.org/10.1007/s00270-008-9489-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-008-9489-z