Abstract
Percutaneous radiofrequency (RF) ablation is a minimally invasive technique used to treat solid tumors. Because of its ability to produce large volumes of coagulation necrosis in a controlled fashion, this technique has gained acceptance as a viable therapeutic option for unresectable liver malignancies. Recently, investigation has been focused on the clinical application of RF ablation in the treatment of lung malignancies. In theory, lung tumors are well suited to RF ablation because the surrounding air in adjacent normal parenchyma provides an insulating effect, thus facilitating energy concentration within the tumor tissue. Experimental studies in rabbits have confirmed that lung RF ablation can be safely and effectively performed via a percutaneous, transthoracic approach, and have prompted the start of clinical investigation. Pilot clinical studies have shown that RF ablation enables successful treatment of relatively small lung malignancies with a high rate of complete response and acceptable morbidity, and have suggested that the technique could represent a viable alternate or complementary treatment method for patients with non-small cell lung cancer or lung metastases of favorable histotypes who are not candidates for surgical resection. This article gives an overview of lung RF ablation, discussing experimental animal findings, rationale for clinical application, technique and methodology, clinical results, and complications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Radiofrequency (RF) ablation is a relatively new minimally invasive technique used to treat solid tumors. It has become a desired image-guided ablative method because of its ability to produce large regions of coagulative necrosis in a controlled fashion. Following recent advances in the RF technology, RF ablation has gained an increasingly important role in the treatment of unresectable hepatic malignancies, and is challenging partial hepatectomy as the treatment of choice for patients with limited hepatic tumor [1,2,3]. Despite the experience with RF ablation of malignant tumors outside the liver being at an early stage of clinical application, recent studies have shown that this technique could offer a valuable treatment option for unresectable lung malignancies [4,5,6,7,8,9,10]. This article reviews the current status of lung RF ablation, discussing experimental animal findings, rationale for clinical application, technique and methodology, clinical results and complications.
Experimental Animal Studies
Experimental studies evaluated feasibility and safety of percutaneous RF ablation of normal pulmonary tissue in rabbits [4] and assessed its effectiveness in the destruction of experimentally-induced pulmonary malignancies [5, 7]. Miao et al. [7] conducted an experimental study in 18 rabbits with pulmonary implantation of VX2 tumors. The rabbits were divided into two groups. Group A (n = 12) was treated with RF ablation and group B (n = 6) received sham operation. All animals in group B died within 3 months after tumor implantation. Tumor eradication was achieved in 9 of 12 rabbits (75%) in group A, of which 4 rabbits survived longer than 3 months free of disease and another 5 rabbits were found free of viable tumor when sacrificed. The 3-month survival rate of RF-treated rabbits was significantly higher (p < 0.01) than that of control rabbits.
We evaluated pathology changes induced by RF ablation in rabbit lung tissue by sacrifying 16 animals at incremental time intervals after the procedure [9] (Figs. 1, 2). Clear-cut, sharply demarcated coagulation necrosis was observed in the animals sacrified 72 hours or more after the procedure. Coagulation necrosis was initially surrounded by a reddish hyperemic rim with inflammatory cells in interalveolar septations. After 15 days, the hyperemic halo was no longer seen, and the lesion was surrounded by a firm, fibrotic, white peripheral rim: in this phase, initial signs of wound organization, with inflammatory cells cleaning necrotic tissue, were observed. In the specimens obtained 30 days after the procedure, regenerating bronchioli were shown, representing a sign of lesion repair. In all cases, lung parenchyma adjacent and far from the thermal lesion did not present any grossly apparent abnormality.
a,b. RF ablation procedure in rabbit lung. The expandable electrode needle is placed in the right upper lobe of the rabbit. After hook deployement, the correct placement of the array is checked by fluoroscopy (a). After having the ablation (b), fluoroscopy shows the presence of a sharp, round opacity in the absence of pneumothorax.
In summary, animal studies showed that lung RF ablation can be safely and effectively performed via a percutaneous, transthoracic approach. Solid lung tumors seemed to be well suited to RF ablation because the surrounding air in adjacent normal parenchyma provides an insulating effect, thus facilitating energy concentration within the tumor tissue. These experimental findings prompted the start of clinical investigation.
Rationale for Clinical Application
Lung cancer is among the most commonly occurring malignancies and is the leading cause of cancer death. Non-small cell lung cancer (NSCLC) comprises approximately 80% of primary malignant tumors of the lung, while most of the remainder are small cell carcinomas [11]. Surgical resection is the treatment of choice for early-stage NSCLC. Unfortunately, patients with NSCLC are frequently poor surgical candidates because of coexistent chronic obstructive bronchopneumopathy or other associated diseases. In addition, NSCLC tends to recur even after successful resection. On the other hand, conventional treatment of nonoperable or nonresectable patients with systemic chemotherapy and external-beam radiation therapy has not been satisfactory in terms of survival outcomes [11].
Lungs are also the second most frequent site of metastatic disease. There have been multiple series documenting survival benefits in patients with pulmonary metastases of favorable histologies who were completely resected as compared to unresectable individuals [12, 13]. However, surgery is frequently precluded by the number and location of metastatic nodules. Moreover, the high risk of recurrence in patients with metastatic disease and the need to remove functioning lung tissue along with the lesions limit the indications for surgery.
Attention has therefore been focused on investigating the effectiveness of RF ablation in achieving tumor destruction in patients with unresectable primary or secondary lung malignancies [7, 9, 14, 15, 16]. RF ablation may prove to be a treatment option for patients with early-stage (T1–T2, N0, M0) NSCLC who are not candidates for surgery. Although RF ablation cannot realistically be expected to achieve the same degree of tumor eradication as complete lobar resection, especially in larger lesions, patients may live longer than if they had not undergone the therapy at all, as it has been shown with limited pulmonary resections [17]. With the same rationale applied to the synergy of postoperative radiation therapy or brachytherapy and limited pulmonary resections, RF ablation may prove to be complementary to chemotherapy and radiation therapy. Hypoxic cells with limited blood flow, such as those found in the center of necrotic tumors, can be resistant to chemotherapy and external-beam radiation therapy. These central hypoxic cells may be more sensitive to RF ablation because of increased cell sensitivity to heat in the hypoxic state and decreased heat dissipation due to poor tumor perfusion [18]. RF ablation may also be suitable for treatment of a small metastatic tumor burden or for palliation of larger lesions that cause symptoms such as cough, hemoptysis, or pain. Because ablation has to be targeted to each individual tumor, this type of therapy is probably best suited for patients with only a small number of slow-growing metastases. A similar approach has been applied to colorectal hepatic metastases whereby treatment is usually limited to four or fewer metastases with the percutaneous approach [3].
Technique and Methodology
Pretreatment Assessment
At our institution, lung RF ablation is performed following treatment protocols approved by the Ethic Committee. Written informed consent is obtained from all patients. Before treatment, a careful clinical evaluation has to be performed, along with any relevant laboratory, imaging, and pulmonary function tests. Inclusion criteria require the patient not to be a surgical candidate. Nonsurgical candidates includes nonoperable and nonresectable cases. RF ablation candidates typically are nonoperable patients with potentially resectable tumors who had been rejected from surgery for comorbidities, especially cardiovascular diseases and chronic bronchopneumopathy resulting in poor pulmonary function. The exclusion of a patient from surgery must be decided by a multidisciplinary team and should be tailored to the individual patient and to the features of the disease. Patients who had previously undergone pneumonectomy must be considered as very high risk patients for RF ablation. These patients were excluded from investigational trials, although in theory they could benefit from RF ablation when discovered with a tumor in the remaining lung. The use of RF ablation in such patients would require an experienced team and proper patient information on the risk associated with the procedure. Patients must have a platelet count greater than 100 × 109 /L and international normalized ratio (INR) of less than 1.5. They must discontinue coumadin, aspirin, and nonsteroidal anti-inflammatory agents at least 5 days prior to the procedure.
The histotype of the tumor is usually confirmed by CT-guided biopsy prior to the ablation. We require hospitalization of the patient and antibiotic treatment is recommended. Usually, a broad-spectrum cephalosporin with a long half-life is administered intravenously for 2 days starting on the day of the procedure, and is followed by an orally administred broad-spectrum antibiotic for 1 week.
Pretreatment CT of the chest is a key examination to determine number, size, and location of the lesions, to carefully evaluate their relationships with major vessels and airways, and to assess the status of the pulmonary parenchyma. According to our ongoing protocols, to be considered suitable candidates for RF ablation, patients are required to have 3 or less lesions per lung, each with a maximum diameter less than or equal to 3 cm. In addition, lesions had to be placed farther than 1 cm from major blood vessels or airways. Other centers, however, have followed wider inclusion criteria, especially with regard to tumor size [16, 18]. Baseline CT provides also the term of reference for posttreatment follow-up studies. To this aim, lung nodule enhancement is studied by performing thin-section CT of the lesion 1, 2, 3, and 4 minutes after the onset of injection [19].
CT Guidance
The procedure is performed following standard rules for CT-guided lung biopsy [20]. The lesion is localized on CT scans, and the grid is superimposed on the CT image at the level of anticipated needle entry (Fig. 3). The skin entry site allowing the shortest, most vertical path that avoids bullae, interlobar fissures, or pulmonary vessels is chosen and a marker is placed on the patient’s skin. The depth of the lesion from the surface is carefully measured. After cleansing the needle entry site with povidine-iodine solution and anesthetizing the skin and subcutaneous tissues with a 2% solution of lidocaine, a small stab incision is made with a scalpel to facilitate needle entry through the skin.
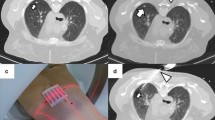
(a–d). CT-guided needle positioning. The patient is in the prone position and the lesion is localized on CT scans. The grid is superimposed on the CT image at the level of anticipated needle entry and the depth of the lesion from the surface is carefully measured (a). Once CT scans have confirmed that the needle tip has actually reached the nodule (b), the deployment of the electrode needle jackhooks within the tumor is assessed both in the axial plane (c) and by using appropriate multiplanar image reconstructions (d).
The electrode needle is advanced through the skin to the proximal edge of the lesion (Fig. 3). Needle advancement still requires careful planning. Even slight degrees of malalignment can cause the tip of the needle to miss the target. This is particularly true for small deep lesions. CT fluoroscopy may be a tool to overcome these difficulties. An increase in resistance is palpable as the needle pierces the lesion. Once CT scans have confirmed that the needle tip has actually reached the nodule, special attention must be placed on verifying the correct placement of the active part of the electrode with respect to the tumor. In fact, unlike in biopsy procedures, a marginal location of the device is not satisfactory. The relationships of the electrode needle with the tumor must be assesed in all the different planes by using appropriate image reconstructions (Fig. 3). When expandable needles with multiple jackhooks are used, it is crucial to check the correct placement of the deployed hooks before starting the ablation.
Anesthesiology Care
Candidates for RF ablation of lung neoplasms—especially those with NSCLC—frequently have a medium-to-high anesthesiology risk. They typically have been rejected for surgery for advanced age, chronic obstructive bronchopneumopathy, or other associated diseases. There is no consensus on the best anesthesiology care for lung RF ablation. Local anesthesia does not produce adequate pain relief for RF thermal ablation. Some centers use general anesthesia and endotracheal intubation. While this approach allows for a good respiration control, it has the disadvantage that assisted ventilation, being performed at a positive pressure, could potentially facilitate the evolution of a pneumothorax caused by the needle puncture. Therefore, other centers, including our own, prefer to perform lung RF under conscious sedation. The association of a hypnotic drug with an ultrashort half-life analgesic drug allows a mild sedation and the patient, who can tolerate the often uncomfortable position, cooperates with the operator and bears the pain induced by treatment. Our standard protocol consists in administering a bolus of ketorolac (0.5–0.8 mg/kg) followed by infusion of propofol (1–2 mg/kg/h) and remifentanil (0.1 µg/kg/min). However, drug posology has to be modulated in relation to the individual patient’s compliance and to the different phases of the procedure. The infusion of the hypnotic drug can be varied between 0.5 and 2 mg/kg/h to achieve a patient sedation that preserves the ability to carry out easy actions. The infusion of remifentanil can be varied between 0.05 and 0.15 µg/kg/min to obtain an optimal analgesia. Attention has to be paid to avoid bolus administration of remifentanil, as this may cause respiratory depression. The procedure is performed under standard cardiac, pressure, and oxygen monitoring with continuous oxygen administration.
RF Ablation Protocol
In RF ablation, frictional heating is created by a high-frequency alternating current that flows from noninsulated electrode tips into surrounding tissue. Pathologic studies have shown that RF treatment produces an irreversible cellular damage that can be demonstrated immediately by the absence of cytosolic and mitochondrial enzyme activity. A definite, well-circumscribed area of coagulative necrosis without intervening areas of viable tumor develops within 72 hours of treatment.
RF ablation of lung malignancies have been performed by using either cooled-tip electrode needles or expandable electrode needles with multiple retractable lateral-exit jackhooks on the tip. Cooled-tip electrodes consist of dual-lumen needles with uninsulated active tips in which internal cooling is obtained by continuous perfusion with chilled saline. They are available either as a single needle or as a cluster array with three needles spaced 0.5 cm apart. Expandable needles have an active surface which can be substantially expanded by hooks deployed laterally from the tip. The number of hooks and the degree of hooks deployment may vary according to the desired volume of necrosis. With either device, ground pads, representing the dispersive electrodes of the system, have to be placed on the patients’s thighs.
At our institution, we use a 150-W generator and 15-gauge, 9-hook expandable electrode needles (StarBurst XL, RITA Medical Systems). The RF generator has multiple temperature displays as well as impedance and power monitoring to assist the radiologist in monitoring and controlling the ablation. The generator is programmed on the “average temperature” mode, and the target temperature is set at 90°C. The power output of the RF generator is initially set at 35 W, and is then increased incrementally to maintain the target temperature. The electrode needle is composed of a stainless-steel hypodermic tubing with a portion exposed and stainless-steel extendible flexible electrodes that deploy out from the trocar tip (Fig. 4). On the handle of the electrode needle there are markings to indicate the amount of electrode array deployment from the trocar. Five temperature sensors are located at the tip of 5 of 9 hooks. Probe-tips temperatures, tissue impedance, and wattage required are graphically displayed and recorded by a dedicated software, installed on a personal computer (Fig. 4).
(a, b). Lung RF ablation performed with expandable needle with nine retractable electrodes (RITA Medical Systems). Thermocouples located at the tip of five of nine electrodes enable real- time monitoring of the effective temperature: combining temperature information with precise location of the electrodes as shown by CT allows for precise delineation of the killing zone encompassing the tumor (a). A dedicated software installed on a portable computer provides graph showing real-time curves of probe-tips temperatures, power output, and tissue impedance throughout the procedure (b).
Maximum power output of the RF generator, amount of electrode array deployment from the trocar, and duration of the effective time of the ablation (time at target temperature) depend on the desired volume of ablation (Table 1). This is established at the beginning of the procedure with the goal to destroy the visible tumor mass plus a 1-cm safety margin of ablation all around. Since the device enables creation of spherical volumes of ablation up to 5 cm in diameter with a single insertion, we selected for our clinical studies lesions that did not exceed 3 cm in the greatest dimension with the goal to achieve a complete single-insertion tumor ablation (Table 1).
When the timer runs out, the RF generator powers off and the automatic, 30-seconds cool-down cycle starts. When the cool-down cycle is completed, the electrodes are rectracted and the “track ablation” can be started. This procedure allows for heating and coagulating the needle track and is aimed at prevening any tumor cell dissemination. One case of difficulty in withdrawing the hooks of the expandable needle at the end of the ablation has been reported [21]. This technical problem never occurred in our experience.
Post-Treatment Assessment and Follow-Up
After completion of the procedure, a single expiratory scan is obtained throughout the thorax and viewed at a narrow window width to detect subtle pneumothorax. The patient is then moved onto a stretcher and positioned in the needle puncture site dependent position, which helps reduce air leak and post-procedural pneumothorax and possibly prevents transbronchial spread of induced alveolar hemorrhage.
A small, asymptomatic pneumothorax is managed conservatively with monitoring of vital signs, administration of nasal oxygen, and follow-up radiographs to confirm stability. On the other hand, a large or symptomatic pneumothorax usually requires the placement of a percutaneous small-bore catheher (Fig. 5). It is generally accepted that a pneumothorax exceeding 30%, even in an asymptomatic patient, requires drainage. However, the decision to drain a pneumothorax must be determined on an individual basis. Because patients tolerate pneumothorax differently depending on underlying lung function, a pneumothorax smaller than 30% may also require drainage.
(a–c). Pneumothorax at CT scans obtained after completion of RF ablation for lung cancer. A small, asymptomatic pneumothorax was detected at the end of the procedure (a). After 10 minutes, the entity of pneumothorax is increased and the patient became symptomatic (b). A percutaneous small-bore catheher is placed to solve the complication (c).
Follow-up studies include clinical evaluation, pulmonary function tests, CT of the chest, and quality of life assessment. The examinations are usually performed 1 month, 3 months, and 6 months after the procedure and at 3-month or 6-month intervals thereafter. Despite the fact that CT is currently the most widely used method for follow-up of treated lesions, MRI and POSITRON emission tomographic imaging may ultimately prove useful as important tests for assessing the adequacy of thermal ablation. Subjects with questionable imaging findings can be biopsied. If there is imaging or cytology evidence of residual or recurrent tumor, the patient can be considered for repeated RF ablation provided that requirements for treatment are still met (Fig. 6).
Clinical Results
In early clinical experiences with lung RF ablation, patients were treated within the framework of feasibility studies, aimed at analyzing safety, tolerability, and local therapeutic effect of the treatment (Table 2).
Dupuy et al. [6] first reported three patients with unresectable lung tumors treated with RF ablation. Zagoria et al. [8] published a case report in which two lung metastatic nodules in a patient with renal cell carcinoma were ablated in the absence of major complications. Steinke et al. [10] described a case of a pulmonary metastasis resected after RF ablation, in which histological proof of complete necrosis was obtained. VanSonnenberg et al. [22] reported their initial clinical experience with 6 patients carrying either primary or secondary unresectable lung tumors, who had exhausted radiation and chemotherapy alternatives. In all cases, extensive necrosis, manifested as no enhancement on the post-procedural contrast-enhanced MRI, was seen. One intraprocedural pneumothorax was encountered and treated intraprocedurally with a small-bore (7 Fr) percutaneous cathether that allowed the procedure to continue.
Lee et al. [16] treated a total of 32 tumors in 30 patients. Tumor size ranged from 0.5 to 12.0 cm in diameter, with a mean diameter of 5.2 cm ± 2.4. RF ablation was performed with two separate underlying rationales. In 10 patients, RF was applied with the intention of achieving definitive therapy. Twenty patients underwent the RF procedure as palliative therapy. Overall, complete necrosis (i.e., ablation of the entire lesion based on evaluation of the Hounsfield unit at CT) was attained in 12 (38%) of 32 lesions, with partially-induced necrosis in the remaining 20 (62%). In the 10 patients in whom RF was applied as a definitive therapy, complete necrosis was achieved in 60% of lesions. In the 20 patients in whom RF was applied as palliative therapy, complete necrosis was attained for only 27%. Most notable, significantly greater complete necrosis was achieved for tumors smaller than 3.0 cm in diameter compared with tumors larger than 3.0 cm in diameter. Specifically, complete necrosis at 6-month follow-up was attained in all six (100%) tumors smaller than 3.0 cm in diameter. Three (10%) major complications were observed in this study. Two large pneumothoraces occurred in patients with central tumors. In addition, an episode of acute respiratory distress syndrome was experienced in a patient who had a recent pneumonia despite systemic antibiotic treatment for longer than a week.
Suh et al. [23] treated 12 patients with 19 unresectable lung tumors (six adenocarcinomas, one large cell carcinoma, two bronchoalveolar carcinoma, four colorectal carcinoma, and six sarcomas less than 50 cm2 in area (range, 0.25–35.00 cm2). RF ablation was well tolerated by all patients. Intraprocedural complications included 12 cases of pneumothoraces 2 required chest tube placement, and 10 were asymptomatic and required no further treatment), 2 cases of pleural effusion, and 2 cases of moderate pain (1 case during and 1 case both during and after the procedure). Mean follow-up was 4.5 months (range, 1–12 months). In the 8 patients with 3-month follow-up, lesion size increased in 2 and remained stable in 6.
Lencioni et al. [24] recently reported the preliminary results of an on-going multicenter trial in which 71 patients with 117 malignant lung tumors 3.5 cm in diameter or smaller were treated. Diagnoses included NSCLC in 27 patients, metastasis from colorectal adenocarcinoma in 34, and metastasis from other primary malignancy in 10. All patients were considered unfit for surgery and had exhausted radiation and chemotherapy alternatives. RF ablation was technically feasible in 70 (98%) of 71 patients. Overall, 116 lesions were treated in 89 treatment sessions. Major complications consisted of pneumothorax requiring treatment (n = 15) and pneumonia (n = 1). CT obtained 1 month after RF ablation showed a round, ground-glass density area encompassing the treated lesion in all cases. Sixty (91%) of 66 lesions in 41 patients who were followed up for 6 months or more after treatment showed no tumor progression on CT. Complete ablation of treated lesions was confirmed by the absence of tumor re-growth over a follow-up period of 1 year or more in 20 patients (Fig. 7). No differences were observed in tumor response rates between patients with NSCLC and those with lung metastases.
(a–d). One-year outcome of RF ablation of NSCLC. Pretreatment CT shows 2.5 cm NSCLC of right lower lobe (a). One month after the RF ablation an area of coagulation necrosis encompassing the treated lesion is depicted, with central cavitation (b). Follow-up CTs obtained 6-months (c), and 1 year (d) after treatment show progressive shrinkage of the area of coagulation necrosis.
Complications
From these preliminary experiences, lung RF ablation seems to be associated with an acceptable rate of adverse events. The most common complication is pneumothorax, which occurred in 20–40% of the interventions, less than half of which required catheter drainage [16, 21, 22, 23, 24]. As in the case of transthoracic needle biopsy, factors most closely associated with the risk of RF-induced pneumothorax are the presence of obstructive airways disease or emphysema. The status of the portion of the lung crossed by the electrode needle is also important to increase the risk of air leak following RF ablation: when possible, the needle path should minimize the amount of diseased lung that must be traversed and avoid crossing large bullae or interlobar fissure. Post-procedural pleurisy and small pleural effusions were observed in patients with pleural-based and peripheral lesions [25]. Most of them were self-limited, but some required aspiration. Productive cough with brown sputum lasting 1–2 weeks after ablation was occasionally observed [25].
Major complications reported include massive hemorrhage, pneumonia, pulmonary abscess, bronco-pleural fistula, and skin burns [15, 16, 21, 22, 23, 24]. Abundant bleeding is a rare complication. While hemorrage around the ablated lesion is quite frequent and almost always self-limited, cases of massive parenchimal bleeding have been reported [26]. Obstructive pneumonia and pulmonary abscess formation are uncommon complications.
Other serious complications related to the needle puncture or to heat injury, however, are possible, especially when lesions close to the heart, main bronchi, or main vessels are treated. A careful assessment of the risks and benefits associated with lung RF ablation has to be made in each individual patient by a multidisciplinary team. Malignant seeding of the needle tract is an exceedingly rare complication of transthoracic needle biopsy. The “track ablation” procedure, performed at the end of RF ablation, should make this possibility even more unlikely despite the larger caliber of the electrode needle compared to that of aspiration needles.
Conclusions
RF ablation is a new, minimally invasive procedure that shows promise for the treatment of primary and secondary lung cancer. Results of pilot clinical trials have shown that this technique can achieve effective and reproducible tumor destruction with acceptable morbidity. Owing to the relatively small number of treated cases and the short follow-up period, no definite conclusion can currently be drawn concerning the potential clinical role of the technique. In fact, no survival benefit associated with the use of RF ablation of lung malignancies has been demonstrated thus far. Nevertheless, it can be predicted that with continued improvement in technology and increasing clinical experience RF ablation could represent a viable alternate or complementary treatment method for patients with non-small cell lung cancer or lung metastases of favorable histotypes who are not candidates for surgical resection.
References
GD Dodd III MC Soulen RA Kane T Livraghi WR Lees Y Yamashita AR Gillans OL Karahan H RRim (2000) ArticleTitleMinimally invasive treatment of malignant hepatic tumors: at the threshold of a major breakthrough RadioGraphics 20 9–27 Occurrence Handle10682768
SN Goldberg GS Gazelle PR Mueller (2000) ArticleTitleThermal ablation therapy for focal malignancies: a unified approach to underlying principles, techniques, and diagnostic imaging guidance AJR Am J Roentgenol 174 323–331 Occurrence Handle1:STN:280:DC%2BD3c7itFOrtA%3D%3D Occurrence Handle10658699
R Lencioni D Cioni C Bartolozzi (2001) ArticleTitlePercutaneous radiofrequency thermal ablation of liver malignancies: technique, indications, imaging findings and clinical results Abdom Imaging 26 345–360 Occurrence Handle10.1007/s002610000194 Occurrence Handle1:STN:280:DC%2BD38%2FhvVShsQ%3D%3D Occurrence Handle11441546
SN Goldberg GS Gazelle CC Compton TC McLoud (1995) ArticleTitleRadio-frequency tissue ablation in the rabbit lung Acad Radiol 2 776–784 Occurrence Handle1:STN:280:DyaK1c%2FotV2juw%3D%3D Occurrence Handle9419639
SN Goldberg GS Gazelle CC Compton PR Mueller TC McLoud (1996) ArticleTitleRadio- frequency tissue ablation of VX2 tumor nodules in the rabbit lung Acad Radiol 3 929–935
DE Dupuy RJ Zagoria W Akerley WW Mayo-Smith PV Kavanagh H Safran (2000) ArticleTitlePercutaneous radiofrequency ablation of malignancies in the lung AJR Am J Roentgenol 174 57–59 Occurrence Handle1:STN:280:DC%2BD3c%2Fpt1Sgtw%3D%3D Occurrence Handle10628454
Y Miao J Yu J Vaninbroukx S Dymarkowski H Zhang G Marchal Y Ni H Bosmans J Yu J Vaninbroukx S Dymarkowski H Zhang G Marchal (2001) ArticleTitleRadiofrequency ablation for eradication of pulmonary tumor in rabbits J Surg Res 99 265–271 Occurrence Handle10.1006/jsre.2001.6208 Occurrence Handle1:STN:280:DC%2BD3MvitFeksw%3D%3D Occurrence Handle11469896
RJ Zagoria MY Chen PV Kavanagh FM Torti (2001) ArticleTitleRadio-frequency ablation of lung metastases from renal cell carcinoma J Urol 166 1827–1828 Occurrence Handle10.1097/00005392-200111000-00050 Occurrence Handle1:STN:280:DC%2BD3MrjvFWltA%3D%3D Occurrence Handle11586236
R Lencioni G Fontanini A Chella L Crocetti C Franchini M Ambrogi A Mussi C Angeletti C Bartolozzi L Crocetti C Franchini M Ambrogi A Mussi C Angeletti C Bartolozzi (2002) ArticleTitlePercutaneous image-guided radiofrequency thermal ablation of the lung Eur Radiol 12 IssueIDSuppl 1 313 Occurrence Handle10.1007/s003300101022
K Steinke JM Habicht S Thomsen M Soler AL Jacob (2003) ArticleTitleCT-guided radiofrequency ablation of a pulmonary metastasis followed by surgical resection Cardiovasc Intervent Radiol 25 543–546
PC Hoffman AM Mauer EE Vokes (2000) ArticleTitleLung cancer Lancet 355 479–485 Occurrence Handle1:STN:280:DC%2BD3c3pslygug%3D%3D Occurrence Handle10841143
U Pastorino M Buyse F. Godehard RJ Ginsberg P Girard P Goldstraw M Johnston P McCormack H Pass JB Putnam RJ Ginsberg P Girard P Goldstraw M Johnston P McCormack H Pard JB Putnam InstitutionalAuthorNamefor The International Registry of Lung Metastases (1997) ArticleTitleLong-term results of lung metastasectomy: prognostic analysis based on 5206 cases J Thorac Cardiovasc Surg 113 37–49 Occurrence Handle9011700
D Kandolier E Kromer H Tuchler A End MR Müller E Wolner F Eckersberger (1998) ArticleTitleLong-term results after repeated surgical removal of pulmonary metastases Ann Thorac Surg 65 909–912 Occurrence Handle10.1016/S0003-4975(98)00019-8 Occurrence Handle1:STN:280:DyaK1c3isVGksg%3D%3D Occurrence Handle9564899
SK Jain DE Dupuy GA Cardarelli Z Zheng TA DiPetrillo (2003) ArticleTitlePercutaneous radiofrequency ablation of pulmonary malignancies: combined treatment with brachytherapy AJR Am J Roentgenol 181 711–715 Occurrence Handle12933465
LJ Herrera HC Fernando Y Perry WE Gooding PO Buenaventura NA Christie JD Luketid (2003) ArticleTitleRadiofrequency ablation of pulmonary malignant tumors in nonsurgical candidates J Thorac Cardiovasc Surg 125 929–937 Occurrence Handle10.1067/mtc.2003.18 Occurrence Handle12698158
Lee JM, Jin GY, Goldberg SN, Lee, YC, Chung, GH, Han, Ym, Lee, SY, Kim, CS (2004) Percutaneous radiofrequency ablation for inoperable non-small cell lung cancer and metastases: preliminary report. Radiology 230: 125–134
RJ Landreneau DJ Sugarbaker MJ Mack (1997) ArticleTitleWedge resection versus lobectomy for stage 1 (T1N0M0) non-small cell lung cancer J Thorac Cardiovasc Surg 113 691–700 Occurrence Handle1:STN:280:ByiB2MfotVM%3D Occurrence Handle9104978
DE Dupuy WW Mayo-Smith GF Abbott T Di Petrillo (2002) ArticleTitleClinical application of radio-frequency tumor ablation in the thorax RadioGraphics 22 S259–S269 Occurrence Handle12376615
SJ Swensen RW Viggiano DE Midthun NL Muller A Sherick K Yamashita DP Naidich EF Patz TE Hartman JR Muhm AL Weaver (2000) ArticleTitleLung nodule enhancement at CT: multicenter study Radiology 214 73–80 Occurrence Handle1:STN:280:DC%2BD3c7gs12hsg%3D%3D Occurrence Handle10644104
DF Yankelevitz M Vasquez CI Henschke (2000) ArticleTitleSpecial techniques in transthoracic needle biopsy of pulmonary nodules Radiol Clin North Am 38 267–279 Occurrence Handle1:STN:280:DC%2BD3c3ivVWqsQ%3D%3D Occurrence Handle10765389
K Steinke J King D Glenn DL Morris (2003) ArticleTitlePercutaneous radiofrequency ablation of lung tumors: difficulty withdrawing the hooks resulting in a split needle Cardiovasc Intervent Radiol 26 583–585
E VanSonnenberg S Shankar KT Tuncali SG Silverman PR Morrison M Jaklitsch (2002) ArticleTitleInitial clinical experience with RF ablation of lung tumors Radiology 225 IssueIDP 291 Occurrence Handle12418464
RD Suh AB Wallace RE Sheehan SB Heinze JG Goldin (2003) ArticleTitleUnresectable pulmonary malignancies: CT-guided percutaneous radiofrequency ablation—preliminary results Radiology 229 821–829 Occurrence Handle14657317
R Lencioni L Crocetti DW Glenn DL Morris RD Suh C Bartolozzi (2003) ArticleTitlePercutaneous radiofrequency ablation of pulmonary malignancies: a prospective, multicenter clinical trial Radiology 229 IssueIDP 437
DE Dupuy SN Goldberg (2001) ArticleTitleImage-guided radiofrequency tumor ablation: challenges and opportunities - Part II J Vasc Interv Radiol 12 1135–1148 Occurrence Handle1:STN:280:DC%2BD3MrjvFWqtw%3D%3D Occurrence Handle11585879
C Vaughn G 2nd Mychaskiw P Sewell (2002) ArticleTitleMassive hemorrhage during radiofrequency ablation of a pulmonary neoplasm Anesth Analg 94 11 Occurrence Handle11772793
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lencioni, R., Crocetti, L., Cioni, R. et al. Radiofrequency Ablation of Lung Malignancies: Where Do We Stand?. CVIR 27, 581–590 (2004). https://doi.org/10.1007/s00270-004-0008-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-004-0008-6