Abstract
We report a successful repair of a ruptured tuberculous pseudoaneurysm of the descending thoracic aorta by endovascular stent graft placement. This procedure is starting to be accepted as an alternative method to surgery, and we review similar cases in the literature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tuberculous pseudoaneurysm of the aorta is a potentially fatal but treatable complication of the disease, so early diagnosis and treatment is necessary. The treatment of aneurysm caused by tuberculous infection should include repair of the damaged vessel wall and use of antituberculous drugs.
We reported a rare vascular manifestation of tuberculosis—a ruptured false aneurysm located at the descending thoracic aorta—in a 69-year-old man without a history of tuberculosis. We also outline the clinical and radiologic features of the disease and discuss the importance of the endovascular treatment with stent graft in selected patients.
Case Report
A 69-year-old man was admitted to our hospital at night with acute chest pain and acute massive hemoptysis with hypotension. His temperature was 37.6°C, respiratory rate 24 breaths/min, blood pressure 85/50 mmHg, and pulse rate 122 beats/min. Laboratory results showed high C-reactive protein and sedimentation levels. The hemoglobin was 10.2 g/dl and the hematocrit level 31.2%. Renal and hepatic function tests and coagulation parameters were normal.
He had history of smoking for 30 years. He was not receiving corticosteroid therapy, and no chest disease was present that had not been known before. He had been complaining about night sweats and a nonproductive cough that had persisted more than 3 months, but he had not sought medical help for that. He denied a family history of tuberculosis or contact with people with tuberculosis.
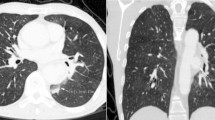
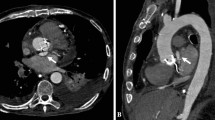
Because of suspected acute aortic dissection, contrast-enhanced multidetector computed tomography (MDCT) of the thorax was performed immediately. It revealed mediastinal widening, massive left hemothorax, and mediastinal lymph nodes. Mediastinal structures were densely surrounded by soft tissue (hematoma), and the trachea and esophagus were displaced anteriorly. There was also a consolidation in the left upper lobe and adjacent to aortic arch (Fig. 1). Computed tomographic (CT) scans revealed a large (7 × 6.8 × 5 cm diameter) saccular contrast-filled structure located next to the medial border of the aortic arch and the descending thoracic aorta between the level from T6 to T9 (Fig. 2). The retained communication with the aortic lumen meant that it was a pseudoaneurysm. The bony structures or the pulmonary vessels showed no abnormality.
On the basis of these clinical and radiologic findings, we suspected infection with Mycobacterium tuberculosis. Blood and sputum samples were taken to confirm the diagnosis, and our patient was tested for the purified protein derivative of tuberculin.
Emergent digital subtraction angiography was performed 2 h after admission with the patient under general anesthesia to confirm the diagnosis, and at the same session, we initiated therapy. We began by controlling arterial access; no severe aorta iliac disease was present. Arcus aortography revealed the orifice of the pseudoaneurysm to be 1.8 cm away from the left subclavian artery origin. We then placed the selective catheter just at the level of the orifice to provide optimal visualization so we could better understand the anatomy of the lesion. It allowed us to see a jet through flow into the aneurysm and to precisely measure the proximal landing zone (Fig. 3). The ascending and descending thoracic aorta below the level of the pseudoaneurysm seemed to be unaffected. The Valiant thoracic stent graft (Medtronic Inc., 36 mm in diameter and 100 mm in length) was positioned well and correctly, without covering the orifice of the left subclavian artery. No endoleak was found immediately after the procedure and at 1 month follow-up CT scans (Fig. 4).
After the intervention, the patient awoke without any neurologic deficits. He was discharged from the hospital 4 days after the operation with antituberculosis medication.
Discussion
Tuberculous aneurysm of the aorta is a rare but potentially fatal complication of tuberculosis if left untreated [1, 2]. Most tuberculous aneurysms are false in type (87%) and saccular in shape (93%) [1]. Previously, researchers had emphasized that the diagnosis of tuberculous aortic aneurysm should always be considered if the aneurysm is false, if it does not involve the ascending aorta, and if it is associated with a contiguous focus of disease on CT scan [3].
Tuberculous aneurysms, whether true or false, may occur anywhere along the arterial system [1, 3]. The thoracic aorta is the most common location because this part is adjacent to the mediastinal lymph nodes [4, 5]. The literature also contains reports of such aneurysms involving the subclavian, carotid, common iliac, hepatic, renal, femoral, and innominate arteries [6, 7]. In our patient, we detected the pseudoaneurysm, located in the thoracic aorta, just distal to the left subclavian artery orifice.
These aneurysms are susceptible to rupture or perforation into adjacent structures, often resulting in fatal exsanguination [2, 8]. The size of the aneurysm does not influence the need for therapy. Therefore, once a pseudoaneurysm has been identified, surgery must be performed without delay, and antituberculosis drugs must be immediately initiated [3, 9].
The pathogenesis of the disease has been explained in previous reports. The process includes the transportation of the bacilli to the vessel wall and the development of the pseudoaneurysm. Tubercle bacilli may reach the aortic wall in one of four ways [3, 10]: (1) the bacilli may implant directly on the internal surface of the vessel wall in patients with miliary tuberculosis; (2) the bacilli may be carried to the adventitia or media by the vasa vasorum; (3) infection may reach the vessel wall by the lymphatics of the vasa vasorum; and (4) the outside of the vessel wall may be affected by direct extension from contiguous infected lymph nodes (tuberculous lymphadenitis), bone (spondylitis), pericardium (pericarditis), paravertebral abscess, or empyema cavities. This last way has been reported as the most common cause (75%) in the literature [3, 9, 10].
We assumed that our patient’s aneurysm was caused by direct extension from the caseous necrotic mass—necrotic lymphadenitis in the aortopulmonary window—into which the aorta had ruptured and penetrated. No vertebral involvement was observed in our patient.
The contiguous focus with caseating necrosis, involving the entire thickness of the aortic wall, results in perforation, either with massive hemorrhage or with the formation of a perivascular hematoma. The latter may become encapsulated and retain communication with the lumen, in which case it is referred to as a false aneurysm or pseudoaneurysm [3, 6]. Conversely, extension of the inflammatory process longitudinally within the aortic wall is more likely to promote the formation of a true aneurysm [6, 11].
Common clinical manifestations include the constitutional symptoms and signs of tuberculosis with or without an aneurysmal mass effect, depending on the location. Tuberculous aortic aneurysm usually manifests as a pulsatile or palpable mass, chest pain, dysphagia, hoarseness, abdominal pain, back pain, and, if complicated, by a fistula, perforations, bleeding, and rupture [1, 3, 5]. Our patient exhibited symptoms related to rupture of the aneurysm: chest pain, hypotension, and hemoptysis. No pulsatile or palpable mass was found at the physical examination. In his history, the additional symptoms such as night sweats and cough made us suspect that tuberculosis could be the possible diagnosis.
The advent of contrast-enhanced MDCT has enabled a noninvasive, first-line method for localizing the site of arterial bleeding. It permits endovascular treatment planning before invasive angiography is initiated to ensure a one-step treatment option for unstable cases [12]. In the present patient, contrast-enhanced MDCT was performed immediately after chest radiography, and then the patient was immediately taken to the interventional radiology department for treatment.
Our review of the literature indicates that many authors state that tuberculous pseudoaneurysm of the aorta can only be cured successfully with both medical and surgical treatment [1, 3, 4, 6, 10]. We agree that the management of tuberculous pseudoaneurysm should include the repair of the lesion on the vessel wall and long-term antimycobacterial drugs to eradicate the source of infection. Surgery is a standard part of the treatment protocol, but the current trend is toward the endovascular method [13–17]. The endovascular approach is already known and has long been performed as an alternative to surgery when treating aneurysms located at the visceral arteries [4] or in cases of Rasmussen aneurysm that cause life-threatening hemoptysis during the course of pulmonary tuberculosis [18, 19]. Transcatheter arterial embolization is becoming a standard first-line treatment option for the management of unstable cases, including patients with acute arterial bleeding from all sources, whereas it was used to be a therapy of last resort, used only when surgical techniques had failed or were considered unfeasible [12].
With the development of thoracic stenting devices, the indications for endovascular treatment for aortic diseases have been expanding [13]. Successful outcomes have been reported with endovascular stent graft placement, which is an alternative and less invasive method to surgical repair of aortic mycotic aneurysms [14, 16].
Endovascular treatment is the best choice in selected patients with thoracic aortic involvement [14, 15] because this technique obviates the increased risk associated with thoracotomy, aortic cross-clamping, and extracorporeal circulation in these critically ill patients [20]. Another good choice for endovascular repair are patients who will greatly benefit from surgical debridement because only a minimal amount of infected tissue exists [14], and patients who also have other diseases and who are not expected to live long [13].
The main problem with the endovascular approach is the impossibility of performing extensive excision and debridement of the infected field, which are the main parts of the surgical strategy. The efficiency of antimycobacterial drugs decreases, and persistent infection occurs [17, 20, 21].
When comparing the potential benefits from minimally invasive endovascular treatment and the higher risk of recurrence of infection, patient condition plays a major role. The surgical procedure should be avoided in debilitated patients because of the high risk of mortality and morbidity. Endovascular treatment may be used as a bridge to a curative (open chest) treatment or as a palliative “destination therapy,” depending on patient condition [17].
In the present case, we considered all the parameters—the patient’s age, his hemodynamic situation, the location of the pseudoaneurysm, the risk of major surgery, and the need for emergency treatment because of acute rupture—and decided on an endovascular approach. Several weeks later, we learned that both the acid-fast stain and the sputum culture were positive for Mycobacterium tuberculosis. Serology for HIV and syphilis were negative. Our patient’s symptoms completely resolved without any recurrence at 8 months’ follow-up.
Although tuberculosis remains primarily a disease of developing countries, the infection has been spreading worldwide during the last decade as a result of increasing resistance to commonly used antimycobacterial drugs and as a result of the continuing increase in infection with human immunodeficiency virus [22–26]. Tuberculous infection should therefore be suspected as an underlying cause of the mycotic aneurysms. Early diagnosis followed by immediate and appropriate treatment is necessary to avoid fatal complications.
The data are too weak for us to suggest or recommend one of the two possible therapies for infected aneurysms of the thoracic aorta. For open repair, we can offer the experiences of the last decades, but with a high rate of mortality and morbidity. Endovascular stent graft placement is becoming accepted as an alternative method to surgery, with favorable outcomes described in current reports. On the other hand, persistent infection remains a major problem because of necrotic tissue, so lifelong antimycobacterial treatment is recommended [17]. The decision should be made on a case-by-case basis. Patient condition plays the major role in deciding the appropriate therapy.
References
Choudhary SK, Bhan A, Talwar S et al (2001) Tubercular pseudoaneurysms of aorta. Ann Thorac Surg 72:1239–1244
Ikezawa T, Iwatsuka Y, Naiki K et al (1996) Tuberculous pseudoaneurysm of the descending thoracic aorta: a case report and literature review of surgically treated cases. J Vasc Surg 24:693–697
Jain AK, Chauhan RS, Dhammi IK et al (2007) Tubercular pseudoaneurysm of aorta: a rare association with vertebral tuberculosis. Spine J 7:249–253
Ohta T (2000) Infected aneurysms. Intern Med 39:875–876
Bukhary ZA, Alrajhi AA (2006) Tuberculous aortitis. Ann Saudi Med 26:56–58
Golzarian J, Cheng J, Giron F, Bilfinger TV (1999) Tuberculous pseudoaneurysm of the descending thoracic aorta. Tex Heart Inst J 26:232–235
Abaskaron M (1986) Multiple pseudoaneurysms in a tuberculous patient. South Med J 79:1582–1584
Uchiyama-Tanaka Y, Mori Y (2006) Miliary tuberculosis with hypercalcemia, and a false abdominal aortic aneurysm, but no pulmonary findings. Intern Med 45:1297–1302
Shikata H, Nagayoshi Y, Takeuchi K et al (2005) Successful surgical treatment of an infrarenal abdominal pseudoaneurysm caused by tuberculosis: report of a case. Surg Today 35:991–995
Long R, Guzman R, Greenberg H et al (1999) Tuberculous mycotic aneurysm of the aorta: review of published medical and surgical experience. Chest 115:522–531
Volini FI, Olfield RC Jr, Thompson JR, Kent G (1962) Tuberculosis of the aorta. JAMA 181:78–83
Keeling AN, Costello R, Lee MJ (2008) Rasmussen’s aneurysm: a forgotten entity? Cardiovasc Intervent Radiol 31:196–200
Loh YJ, Tay KH, Mathew S et al (2007) Endovascular stent graft treatment of leaking thoracic aortic tuberculous pseudoaneurysm. Singapore Med J 48:193–195
Steichen O, Pellerin O, Frank M et al (2007) Endovascular repair of a tuberculous aortic false aneurysm. Rev Med Interne 28:196–198
Liu WC, Kwak BK, Kim KN et al (2000) Tuberculous aneurysm of the abdominal aorta: endovascular repair using stent grafts in two cases. Korean J Radiol 1:215–218
Smith JJ, Taylor PR (2004) Endovascular treatment of mycotic aneurysms of the thoracic and abdominal aorta: the need for level I evidence. Eur J Vasc Endovasc Surg 27:569–570
Labrousse L, Montaudon M, Le Guyader A et al (2007) Endovascular treatment of a tuberculous infected aneurysm of the descending thoracic aorta: a word of caution. J Vasc Surg 46:786–788
Jayet PY, Denys A, Zellweger JP et al (2004) Successful embolization of Rasmussen’s aneurysm for severe haemoptysis. Swiss Med Wkly 134:705–706
Remy-Jardin M, Wattinne L, Remy J (1991) Transcatheter occlusion of pulmonary arterial circulation and collateral supply: failures, incidents and complications. Radiology 180:699–705
Ting AC, Cheng SW, Ho P et al (2005) Surgical treatment of infected aneurysms and pseudoaneurysms of the thoracic and abdominal aorta. Am J Surg 189:150–154
Hatem CM, Kantis GA, Christoforou D et al (2002) Tuberculous aneurysm of the descending thoracic aorta. J Thorac Cardiovasc Surg 123:373–374
Forbes TL, Harris JR, Nie RG, Lawlor DK (2004) Tuberculous aneurysm of the supraceliac aorta. Vasc Endovasc Surg 38:93–97
Gouny P, Valverde A, Vincent D et al (1992) Human immunodeficiency virus and infected aneurysm of the abdominal aorta: report of three cases. Ann Vasc Surg 6:239–243
Falkensammer J, Behensky H, Gruber H et al (2005) Successful treatment of a tuberculous vertebral osteomyelitis eroding the thoracoabdominal aorta: a case report. J Vasc Surg 42:1010–1013
Barnes PF, Barrows SA (1993) Tuberculosis in the 1990 s. Ann Intern Med 119:400–410
Raviglione MC, Snider DE Jr, Kochi A (1995) Global epidemiology of tuberculosis: morbidity and mortality of a worldwide epidemic. JAMA 273:220–226
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dogan, S., Memis, A., Kale, A. et al. Endovascular Stent Graft Placement in the Treatment of Ruptured Tuberculous Pseudoaneurysm of the Descending Thoracic Aorta: Case Report and Review of the Literature. Cardiovasc Intervent Radiol 32, 572–576 (2009). https://doi.org/10.1007/s00270-008-9456-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-008-9456-8