Abstract
Ascending aortic pseudoaneurysms are a rare but potentially life-threatening complication of aortic root or cardiac surgery. Surgical repair is established as first-line treatment; however, patient comorbidities, technical considerations, and anatomic limitations often preclude patients from repeat surgery, thus necessitating alternative approaches. Here, we present a case of coil embolization of an ascending aortic pseudoaneurysm via a transapical approach in a particularly complex scenario where percutaneous and peripheral access was technically unfeasible.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
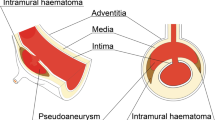
Pseudoaneurysms of the ascending aorta can occur as a result of infection, trauma, and autoimmune disease; however, they are most commonly seen in those with the previous cardiac surgery [1]. The incidence of ascending aortic pseudoaneurysms is as low as 0.5% in the general population, but as high as 13% in those with prior cardiac surgery [2, 3]. If left untreated, pseudoaneurysms of the ascending aorta can be a cause of significant morbidity and mortality, with potential for hemorrhage, rupture, fistulization, or serving as a source of recurrent infection or embolism [2]. Given their unpredictable progression, aggressive management is often recommended, of which surgery is the established first-line treatment [4]. However, in those with significant comorbidities or contraindications to surgery, endovascular treatment can be performed. Most frequently, this has involved the off-of-label use of septal closure devices, vascular plugs (with or without concomitant coil embolization), or stent grafts. A few case reports exist for the use of coil embolization alone, either via a percutaneous approach or by peripheral vascular access. Here, we present a case of coil embolization of an ascending aortic pseudoaneurysm via a transapical approach.
Case Report
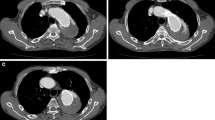
A 62-year-old man was referred to interventional radiology for endovascular treatment of a postsurgical ascending aortic pseudoaneurysm. The patient had a complicated course in hospital involving two open repairs of a recurrent aortic root abscess along with replacement of his aortic valve and repair of his mitral valve. Following his most recent repair, a CT angiogram revealed a 22 × 8 mm, narrow-necked (2 × 2 mm) pseudoaneurysm had developed along the aortotomy suture line posterior to the aortic valve, between the left coronary cusp and left atrium, and in close proximity to the left main coronary artery (Fig. 1). Given the risk of rupture and of left main coronary artery occlusion, semi-urgent treatment was deemed necessary. With the patient’s two recent sternotomies, it was likely that the left ventricular apex would be adherent to the anterior chest wall and a third operation would incur too much risk. It was therefore decided that an endovascular approach would be more appropriate.
The narrow 2 × 2 mm neck of the pseudoaneurysm notably favored a coil embolization approach as it would allow for dense coil packing while minimizing the risk of coil dislodgement. Its location posterior to aortic valve precluded direct percutaneous pseudoaneurysm access; thus, the aneurysm would have to be accessed directly via the left ventricular outflow tract. A transfemoral approach was first attempted. Since the pseudoaneurysm neck arose from the suture line, inferior to the valve itself, there was no need to protect the left main coronary artery. However, its origin below the valve added additional difficulty in obtaining stable positioning within the aneurysm. Despite prolonged efforts using a variety of catheter shapes and wire combinations, there was insufficient stability to cannulate the neck and the procedure was aborted. It was felt that additional approaches from above, such as a transradial approach, would likely fail for a similar reason. Therefore, in consultation with the cardiac surgery team, it was decided that the patient would be brought back for a transapical approach (Fig. 2).
Fluoroscopic images demonstrating the transapical coil embolization of the LVOT pseudoaneurysm. A Transapical approach setup: a = aortic valve, m = mitral valve, e = transesophageal echocardiography probe, c = catheter entering through the left ventricular apex. B Contrast injection demonstrating the pseudoaneurysm sac (arrow). C Microcatheter cannulation of the pseudoaneurysm sac. D Advancement of the 5F catheter into the sac and initial coiling. E Mid-procedural acquisition demonstrating loosely packed coils within the pseudoaneurysm. F Complete embolization demonstrating dense, homogenously packed coils
With the patient under general anesthesia, a left mini-thoracotomy was performed. The apex of the left ventricle was punctured, and a 7-French sheath was inserted. Once access was secured, 100 IU/kg heparin IV was administered. A 60-cm 7-French cardiology guide catheter (Mach1 MP2, Boston Scientific, Marlborough, MA) was then inserted through the sheath, followed by a 5-French 45 degree angled diagnostic catheter (Glidecatheter, Terumo Medical, Somerset, NJ, USA). This platform allowed greater support than a smaller bore system, particularly as cardiac motion could not be stopped. The sac was then successfully cannulated using a Fathom-16 microwire (Boston Scientific, Marlborough, MA, USA) and Lantern microcatheter (Penumbra Inc, Alameda, CA, USA) followed by the 5-French guide catheter. The base catheter was occlusive within the neck of the aneurysm, negating the risk of coil migration. Its tip remained protected by the 5-French guide catheter and microcatheter combination, and with at least 1 cm between the catheter tip and pseudoaneurysm wall to minimize the risk of perforation. The pseudoaneurysm was then coiled using a combination of SMART and RUBY coils (Penumbra Inc, Alameda, CA, USA), ranging between 3 and 18 mm in diameter and 6 and 60 cm in length, resulting in approximately 35% packing density. Post-embolization angiography confirmed cessation of flow within the pseudoaneurysm sac, which was confirmed by transesophageal echo Doppler. Anticoagulation was then reversed with protamine sulfate, all equipment was removed, and the ventricular access site was closed. Including patient preparation, the procedure lasted 5 h and 20 min.
Post-procedural CT angiogram showed no filling of the pseudoaneurysm, which was again confirmed with a one-month follow-up CT angiogram (Fig. 3). The patient was eventually discharged but was lost to imaging follow-up after returning to his hometown. Unfortunately, he passed away from unrelated medical disease six months post-procedure.
Discussion
Postoperative pseudoaneurysms of the ascending aorta can occur at any site of aortic manipulation. Most commonly, they occur at sites of anastomotic suturing or cannulation [4]. Surgical repair carries a high risk of mortality, ranging between 8.6 and 41%, most often due to pseudoaneurysm rupture upon sternal re-entry, coronary embolism, or a low cardiac output state postoperatively [5]. Endovascular approaches are sometimes required as an alternative due to significant patient comorbidities or due to anatomic reasons, such as a pseudoaneurysm located immediately deep to the sternum.
The choice of embolization technique is crucial and careful consideration of all options is necessary for each case. Septal occluder devices and vascular plugs have become the most common form of endovascular treatment in the published literature [6]. A benefit of occluder devices is the wide array of sizes available, which can allow treatment of some larger neck pseudoaneurysms. However, the long-term efficacy of these devices has been called into question due to the risk of device migration, as the friability of the pseudoaneurysm tissue may limit stable device position [5].
Commercially available stent grafts specific to the ascending aorta are limited by the relatively clustered anatomy about the ascending aorta, risking occlusion of the coronary ostia, arch vessels, or bypass grafts. While stent grafts can be technically feasible, the correct patient, pseudoaneurysm type, and location must be appropriately selected [7]. In our case, the aneurysm was at the level of the valve; thus, a stent graft could not be appropriately placed with the necessary 2-cm landing zone.
Two case reports describe the use of percutaneous intra-arterial thrombin injection for ascending aortic pseudoaneurysms, one of which was complicated by transient hemiplegia as a result of carotid artery thrombosis [8]. Other liquid embolic agents would likely carry a similar risk of non-target embolization, particularly within the ascending aorta, where pseudoaneurysms have a potential for higher internal velocity and turbulent flow.
Coil embolization is ideal for saccular pseudoaneurysms with narrow necks where dense packing of coils can be achieved with low risk of dislodging. Detachable coils provide an additional benefit as they can be easily retrieved if they appear unstable or poorly sized. Coils can be used as the sole embolotherapy, but are more commonly used combination with stent grafts or occluder devices [6]. The use of coils alone is limited to a few case reports [9,10,11,12,13]. Obtaining stable positioning can be a challenge, particularly when the neck arises below the valve, as we learned from our transfemoral attempt. One published case demonstrates the use of robotic assistance for additional stability via a transfemoral approach [13].
Hibino et al. [14] demonstrated a combined transapical and transfemoral approach in a case where the neck of the pseudoaneurysm arose from a sinus of Valsalva and was embolized using a combination of an Amplatzer Vascular Plug II (St Jude Medical, Saint Paul, MN, USA) and coils. The vascular plug eventually failed requiring open repair; however, the case demonstrated the potential of the transapical approach for lesions arising near the aortic root. This may be particularly true in cases such as ours, where the pseudoaneurysm neck is located inferior to the valve itself. Given the easier accessibility and proximity, the transapical approach should be considered a viable alternative for pseudoaneurysms of the ascending aorta.
Conclusion
Here, we present the feasibility of the transapical approach for coil embolization, which may be of particular use in cases where peripheral access or percutaneous routes are not achievable.
References
Sullivan KL, Steiner RM, Smullens SN, Griska L, Meister SG. Pseudoaneurysm of the ascending aorta following cardiac surgery. Chest. 1988;93(1):138–43.
Katsumata T, Moorjani N, Vaccari G, Westaby S. Mediastinal false aneurysm after thoracic aortic surgery. Ann Thorac Surg. 2000;70(2):547–52.
Mesana TG, Caus T, Gaubert J-Y, Collart F, Ayari R, Bartoli J-M, et al. Late complications after prosthetic replacement of the ascending aorta: what did we learn from routine magnetic resonance imaging follow-up? Eur J Cardiothorac Surg. 2000;18(3):313–20.
Atik FA, Navia JL, Svensson LG, Vega PR, Feng J, Brizzio ME, et al. Surgical treatment of pseudoaneurysm of the thoracic aorta. J Thorac Cardiovasc Surg. 2006;132(2):379–385.e1.
Quevedo HC, Alonso A. Endovascular therapy for ascending aorta pseudoaneurysm. Cardiovasc Revasc Med. 2016;17(8):586–8.
Quevedo HC, Santiago-Trinidad R, Castellanos J, Atianzar K, Anwar A, Rafeh NA. Systematic review of interventions to repair ascending aortic pseudoaneurysms. Ochsner J. 2014;14(4):576–85.
Preventza O, Henry MJ, Cheong BYC, Coselli JS. Endovascular repair of the ascending aorta: when and how to implement the current technology. Ann Thorac Surg. 2014;97(5):1555–60.
Lin PH, Bush RL, Tong FC, Chaikof E, Martin LG, Lumsden AB. Intra-arterial thrombin injection of an ascending aortic pseudoaneurysm complicated by transient ischemic attack and rescued with systemic abciximab. J Vasc Surg. 2001;34(5):939–42.
Chapot R, Aymard A, Saint-Maurice J-P, Bel A, Merland J-J, Houdart E. Coil embolization of an aortic arch false aneurysm. J Endovasc Ther. 2002;9(6):922–5.
Miguel B, Camilleri L, Gabrillargues J, Macheda B, Kubota H, Ravel A, et al. Coil embolization of a false aneurysm with aorto-cutaneous fistula after prosthetic graft replacement of the ascending aorta. Eur J Radiol. 2000;34(1):57–9.
Barbetakis N, Xenikakis T, Efstathiou A, Fessatidis I. Percutaneous coil embolisation of a false aortic aneurysm following coronary surgery and mediastinitis. Hell J Cardiol. 2007;48(4):246–8.
Arain N, Bryant R, Ameduri R, Gruenstein D, Braunlin E, Joyce L, et al. Successful coil embolization of a large ascending aortic pseudoaneurysm following explantation of the EXCOR pediatric ventricular assist device in a patient with acute fulminant myocarditis. World J Pediatr Congenit Heart Surg. 2010;1(2):259–61.
Lu T, Owji S, Chinnadurai P, Loh TM, Schwein A, Lumsden AB, et al. Robotic-assisted coil embolization of ascending aortic pseudoaneurysm. Ann Thorac Surg. 2016;102(5):e451–3.
Hibino M, Katada Y, Shibayama K, Obunai K, Watanabe H, Kawano Y, et al. Recurrent aortic root pseudoaneurysm after transcatheter occlusion—a word of caution. J Card Surg. 2018;33(4):190–3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dennis Parhar, Gerald Legiehn, John Chung, Anson Cheung, and Michael Janusz declare that they have no conflict of interest. Darren Klass is a consultant for Cook Medical and consultant for Merit Medical, and has a research grant with Biolife (StatSeal).
Human and Animals Rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Parhar, D., Klass, D., Legiehn, G. et al. Transapical Coil Embolization of a Postsurgical Ascending Thoracic Aortic Pseudoaneurysm. Cardiovasc Intervent Radiol 42, 1500–1504 (2019). https://doi.org/10.1007/s00270-019-02291-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-019-02291-w