Abstract
Purpose
To quantify the influence of angiography table height on patient and angiographer irradiation, as compared with other routine protective measures such as the use of protective shields hanging at the tableside and from the ceiling of angiography suites.
Methods
An experimental study was carried out in which a phantom (substitute for a human body) placed on the angiography table was irradiated by pulsed fluoroscopy. Entrance exposure rates were measured at the phantom surface (surrogate of patient skin exposure by incident X-ray beam) and at 60 cm from the phantom (analog to angiographer skin exposure by scatter). Exposure rates were measured at levels corresponding to the knees, testes, waist, xyphoid appendix, shoulders, and eyes of an angiographer 178 cm tall. Measurements were repeated at angiography table heights of 85, 95, 105, and 110 cm from the floor, with and without protective shields.
Results
Moving the table from its highest to lowest position increased by 32% the phantom entrance exposure but decreased scatter to the angiographer. Scatter to the angiographer could be reduced most by using the protective shields (30–105 times less), but low table heights provided relatively more important protection (412–1121 μSv/hr reduction, or 15–72% scatter reduction) when shields were not used (e.g., for unprotected regions of the angiographer’s body such as the hands).
Conclusion
Working at lower table heights provides a little additional protection to exposed body parts of angiographers, at the cost of somewhat higher patient exposure. Although small, this incremental protection could be clinically relevant in the long term. The choice of table position should be a compromise based on multiple factors, including at least patient exposure, scatter to angiographers, and angiographer comfort.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Many interventional radiology (IR) procedures can result in clinically significant radiation doses to patients and to the physicians and paramedical staff performing them. The radiation protection of primary operators is of particular concern, given the repetitive nature of operator exposure during IR procedures and potential long-term consequences.
Multiple factors can help reduce operator exposure to X-rays [1–3]. Some can produce global reduction of scatter within the IR suite, such as “low patient dose” mode, the use of pulsed fluoroscopy instead of continuous mode [4, 5], and digital magnification [6]. Others provide local protection and include notably (a) the avoidance of direct exposure of the operator’s hands in the primary beam and (b) various attempts to attenuate the source of scatter (i.e., the irradiated patient volume) such as working away from the patient’s (entrance) skin surface (where the incident primary X-ray beam enters and most of the scatter occurs) [7], increasing the thickness of operators’ lead aprons [8], and the use of several other protective devices [8–12].
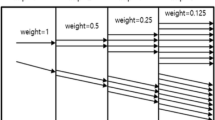
The influence of some of these local factors on operator irradiation has been quantified previously. For example, caseload and apron thickness seem more important quantitatively than case mix, experience, lead apron type, additional lead shielding, and several other fluoroscopy features [8, 13]. In addition, there remain other local parameters that can influence operator irradiation, including notably the location of different protective shields that are integral components of the angiographic suite, and the examination table height at which patients are positioned during IR procedures (Fig. 1). While the protective effect of shields is well documented, the effect of table height on patient and operator exposure and its quantitative importance may be intuitively less straightforward to IR staff. The aims of the present experimental study were (1) to quantify the relative influence of these factors on operator irradiation and (2) to identify regions of high scatter level under variable working conditions.
Materials and Methods
Theoretical Considerations on Table Height
On current angiographic tables, the use of isocentric geometry provides the advantage of less repositioning of the patient when the gantry (C-arm) is rotated. In real life, however, table position may change between patients and, for a given patient, during a procedure. Several points need to be taken into account to understand variations in patient and operator exposure caused by different tabletop heights (Fig. 1).
First, the primary source of scatter is the volume of interest imaged/explored in the patient. This volume of interest varies and depends largely on (1) the “active” phosphor size of the image intensifier, or the “active” imaging area being selected on a flat panel detector imaging system, and (2) the imaging geometry. In both “low” and “high” table positions, this volume of interest irradiated by the primary (incident) X-ray beam remains nearly the same, resulting in similar sizes of scatter sources from the patient to the operator (also referred to below as the angiographer) (i.e., volume A = volume B or almost). Thus, the fact that the diverging geometry of the primary X-ray beam is wider at lower table positions (i.e., angle α > angle β in Fig. 1, posteroanterior projection) is not of concern here. Therefore, we will only consider a constant volume of interest in the present study.
Second, another variable parameter associated with table height changes is the variation in source-to-object distance (SOD) between low and high table positions. The magnitude of this variation is relatively small (maximal difference: 25 cm in the equipment used in the present experiment). Therefore, differences in energy loss of the primary X-ray beam in room air along this 25 cm distance are likely minimal. On the other hand, the longer SOD associated with high table positions could result in a lower patient skin entrance dose because of the “inverse square of distance law.” In fact, the flat panel (or the image intensifier) requires the same input exposure (rate) to maintain satisfactory image brightness and signal level. As the source-to-image receptor distance (SID) is increased and the input exposure rate to the image receptor decreases, the automatic brightness control circuit will, in order to maintain the proper signal level, send command signals to the generator to vary the tube potential, the tube current, and the pulse width, in accordance with the fluoroscopic operation logic. Therefore, the system will automatically adjust patient irradiation parameters as needed to provide appropriate image signals to the fluoroscopy monitor.
Lastly, if the SOD remains unchanged while the SID is increased (air gap), one can expect a higher patient skin entrance dose, again due to the automatic brightness control circuit of the imaging system. This variation in examination geometry is beyond the scope of the present experiment, and for the sake of simplicity no air gap geometry was included in this study. Overall, the relative spatial location of the scatter source (patient), the operator, and the protective shields available in the angiography suite need to be taken into consideration to explain possible differences in patient and operator exposure at different table heights and in different working conditions [7].
Experimental Setting
An experimental dosimetric study was carried out by irradiating a phantom placed on a standard angiography table (Fig. 2). The phantom consisted of 10 pieces of 2.54 cm nominal thickness polymethylmethacrylate (PMMA) plastic and was irradiated with pulsed fluoroscopy (15 frames per second) in the posteroanterior projection using a field of view 32 cm wide and a constant air gap of 3.5 cm. The phantom (patient’s surrogate) entrance exposure rate was measured with an ionization chamber (Keithley Model 96035 Ionization Chamber connected to a TRIAD TnT Dosimeter Model 35035) as depicted in Fig. 2. The scattered radiation to the operator was measured with a survey meter designed for scattered radiation measurements (Keithley Model 36155 Survey Meter). In order to account for the response time (4 sec) of the survey meter, the minimum measurement time was at least 15 sec. The operator position was chosen at 60 cm from the phantom center (measured in the table plane (i.e., the coronal plane of an imaginary supine patient), parallel to the floor) where the operator would be standing during actual clinical cases. This position was chosen perpendicular to the lateral aspect of the rectangular phantom (i.e., not along its diagonals), in an attempt to mimic a percutaneous transhepatic biliary procedure from a right-sided approach. In that position, exposure rate was measured at six different heights, corresponding approximately to the knees (50 cm above floor), testes (80 cm), waist (100 cm), xyphoid appendix (120 cm), shoulders (140 cm), and eyes (160 cm) of an operator 178 cm tall.
Two parameters related to the angiographic suite equipment were modified during successive experiments. First, dosimetric measurements were obtained with and without the use of the protective shields that are integral components of this angiographic suite, including both the lead drapes/shielding hanging from supporting rails at the tableside and the overhead protective shield hanging from the ceiling suspension system (Fig. 2). The principle behind these modifications was to simulate three possible scenarios: (a) when none of the available protective apparatus is employed (arrangement A, “worst conditions”), (b) when only the lead drapery hanging from the tableside is utilized (arrangement B, “suboptimal conditions”), and (c) when all available protective apparatus is taken advantage of (arrangement C, “optimal conditions”). Second, table height was modified using SIDs of 90, 100, 110, and 115 cm (corresponding to table heights of 85, 95, 105, and 110 cm from the floor, respectively) (Fig. 2).
Results
Dosimetric measurements obtained by the present experiment are detailed in Table 1 and Fig. 3 and 4. In the highest table position, the X-ray production system automatically increased the intensity and exposure time in pulse width of the incident X-ray beam (up to 27% more mA and 16% wider pulse width, respectively) while maintaining a constant voltage, in order to maintain image quality. At the same time, the entrance exposure rate to the phantom was 24% lower at the highest table position than at the lowest table position due to the inverse square of distance law.
Bar diagram of exposure rates measured in the worst conditions (no shield). The upper curve represents the integral function curve passing through the right-hand (dark) columns (SID =115 cm) at each operator body level studied. The lower curve corresponds to the left-hand (light) columns (SID = 90 cm). The difference in the areas under these curves is obvious.
Bar diagram of exposure rates measured in “optimal” conditions (note the smaller y-axis scale than in Fig. 3). The maximal exposure measured in this graph (highest column at 160 cm) corresponds to high scatter to the operator’s eyes (160 cm height from floor) when the table is in the lowest position (SID = 90 cm) (i.e., the “optimal” conditions are still perfectible). This may happen when the operator is tall enough to be higher than the upper aspect of the ceiling screen, so that scatter from the patient can directly reach the angiographer’s eyes along a straight line (arrow) passing between the flat panel detector (image intensifier) and the ceiling screen. A higher position of the ceiling screen would better protect the operator’s face but introduce a gap below it. A bigger ceiling screen would be less convenient for the operator.
For the operator (angiographer), maximal protection observed was provided by using the ceiling-mounted and tableside shields. Without any protective shielding (Fig. 3), the scattered radiation to the operator is in the range 272–3054 μSv/hr and is maximal at the level of the pelvis. The use of the tableside lead drape alone (1) decreased dramatically the scattered radiation to the angiographer’s pelvis and legs (39–111 μSv/hr) corresponding to measurement heights of 50 cm (knees) and 80 cm (testes), while it (2) showed no effect for heights of 140 cm (shoulders) and 160 cm (eyes). At measurement heights of 100 cm (waist) and 120 cm (xyphoid appendix), there were some reductions in scattered radiation as well. This is due primarily to the fact that the flat panel assembly is blocking some of the scattered radiation; the shorter the SID the more reduction of scattered radiation is observed. Use of both protective shields (Fig. 4) provided optimal protection (15–58 μSv/hr). The radiation scattered to the angiographer was decreased by a factor of 30–105 compared with the worst-case scenario (arrangement A) where no protective shielding is utilized (except for the eye level at the lowest table position, where the reduction was 5-fold only, due to interposition of the flat panel assembly between the phantom and the operator’s head). However, this protection applies only to non-exposed parts of the operator’s body hidden behind the protective shielding. Therefore, our so-called suboptimal conditions (i.e., using the lower, tableside lead drape alone) better reflect what happens to the angiographer’s unprotected hands and arms while working on the patient, while Fig. 4 (i.e., optimal conditions: both shields used) is more suited to describing scatter to the rest of the operator’s body (head, neck, torso, legs).
The influence of table height on operator irradiation was more subtle and is illustrated in Fig. 4, which is similar to Fig. 3 but with a much smaller scale along the y-axis. In general, operator exposure was less when the table was placed lower: as illustrated in Fig. 4, at each level of the operator’s body, the height of the columns decreases progressively from right to left. That is, scatter was reduced when going from a SID of 90 cm (light columns) to a SID of 115 cm (dark columns). The magnitude of the change in operator exposure varied depending on the clinical scenario and a measurement variance of ± 10%; i.e., reproducibility in measurement locations. However, it can be said that:
-
(1).
When both the tableside and ceiling shields were used (i.e., optimal conditions), lowering the table from its highest to lowest position resulted in small decreases in scattered radiation to the operator, ranging from 9 (= 24 − 15) to 40 (= 58 − 18) μSv/hr depending on the level of the operator’s body being considered (Table 1). This corresponded to scatter reductions of 26% (pelvis) to 69% (eyes). Most of the scattered radiation was observed at the level of the angiographer’s lower body (knees, testes and pelvis), which is closer to the location of the phantom at low table heights. It is of note that the scattered radiation coming from the phantom tended to be progressively blocked by the flat panel detector (image intensifier) assembly as the table was lowered, which provided relative protection to the operator’s eyes. There was one main exception, however: when the table is at its lowest possible level, the head and eyes of a tall angiographer can be inadvertently located higher than the upper aspect of the ceiling screen due to the limited size of that screen (Fig. 4) and are therefore unprotected. Thus scatter from the phantom could directly reach the operator’s eyes by passing in a line between the flat panel detector and the ceiling screen. This can explain the highest exposure value observed with arrangement C (highest column at 160 cm in Fig. 4). While this is an that indication the ceiling screen should have been positioned higher to protect the eyes, it also points to the fact that a taller screen may be necessary for a taller angiographer.
-
(2).
When only the tableside drape is used (i.e., suboptimal conditions), table height became more important quantitatively than in optimal conditions: placement of the angiography table in the lowest position resulted in larger decreases in exposure rate to the operator’s upper body parts, ranging from 278 to 1034 μSv/hr (=1383 − 1105 and 1282 − 248, respectively). This corresponds to scatter decreases ranging from 20% to 81%. Due to the protection from the tableside drape, smaller decreases in exposure rate were observed with respect to the angiographer’s pelvis and legs, ranging from 13 to 62 μSv/hr (= 52 − 39 and 111 − 49, respectively).
-
(3).
When no shielding is present (worst conditions), the influence of table position becomes even more important, again with decreases in the operator’s exposure rate obtained when a low table height is chosen. These decreases ranged from 412 to 1121 μSv/hr (= 2,703 − 2,291 and 2,302 − 1,181, respectively), or from 15% at the level of the operator’s knees to 72% at his or her eye level.
Discussion
The present experiment investigated the influence of the height of the angiography table on patient entrance exposure and scattered radiation to the interventional radiologist and its relative importance compared with that of the protective apparatus available during IR procedures. This study verified the well-known highly protective effect of the protective shieldings. By using such shields, operators can reduce scatter to most regions of their body by a factor of 30–105 times. This is consistent with the rule of thumb saying that less than 3–5% of the scatter radiation passes through 0.5 mm lead-equivalent lead aprons and protective apparatus. Under shielding arrangement C with the full armament of protective apparatus operators received less scatter when the table was positioned lower, although at the cost of a higher patient skin entrance dose (6.3 mGy/min difference, a 32% increase compared with the highest table level). Because of its small magnitude, the protection provided by working at a low table height is relevant mostly for non-protected areas of the operator’s body, such as their hands working close to the field of view.
Thus, a compromise must be found between these conflicting trends. From the patient’s perspective, the effects of several operational parameters on patient skin dose have been shown to be multiplicative [4]. From the angiographer’s point of view, exposure to the scattered radiation during IR procedures is repetitive in nature. Although our data suggest that table height is of minor importance quantitatively, this experiment was short and cumulative exposure to scatter may potentially result in dire consequences in the long term. Additionally, other factors may influence the compromise, such as operator comfort: an appropriate table position is also one that does not cause back pain in the long term, and this may be important for taller operators. We are not advocating using a low table level in each and every case: other considerations need to be taken into account.
It is of note that the image intensifier may sometimes be interposed, at least partially, between the patient and the angiographer’s head and therefore can actually be shielding the operator’s head from the scatter. Thus, lowering the flat panel detector is not only useful to reduce the air gap and geometrical unsharpness, but may also help to protect the operator’s eyes.
Table height seems to be a relatively variable parameter in real life. In our clinical experience (from a random, non-consecutive selection of 28 procedures performed by 14 operators over 3 months), IR procedures were typically performed at table heights ranging between 84 and 107 cm (i.e., a 23 cm range). This is similar to the full range of table positions tested in the present experiment (25 cm). To put these findings in a broader context, we also reviewed retrospectively our experience with de novo percutaneous nephrostomy tube placements over a 3 year period (2001–2004): the mean total fluoroscopy time during the procedure and its 95% confidence interval were 11.8 ± 2.0 min (range 1–42 min). Thus, on average, an angiographer performed 5.1 nephrostomies before reaching 1 hr of fluoroscopy time (60/11.8 min). By extrapolating these data to the measurements from the present experiment, a primary IR operator would, on average, receive 1243 μSV of scatter on his or her shoulders after 1 hr of fluoroscopy (5.1 nephrostomies) performed at the highest table position. Alternatively, he or she would receive the same amount of scatter after 11.0 nephrostomies ((1243/572) × 5.1) performed at the lowest table height. That is, one could perform at least twice the number nephrostomies at a low compared with a high table position before reaching that given amount of scatter. This assumes, however, that a high table height would not cause sufficient discomfort to slow down the speed of performing the procedure and result in an increased overall procedure time, which stresses again the importance of an ergonomic position tailored to each individual operator.
There are several other relevant practical implications of this investigation. First, it shows that table height becomes an important parameter when working conditions are not optimal in terms of shield protection. This may become important (1) when the ceiling shield is inadvertently misplaced outside the line of sight between the patient and the operator, for example during difficult procedures or with inexperienced or distracted angiographers. The ceiling shield is often cumbersome and, therefore, sometimes neglected during interventions (Fig. 4). The results found here underline the need to use protective shields whenever possible. Second, even in optimal conditions, with perfect positioning of all protective apparatus, the upper extremities of the operator may remain exposed directly to scatter from the patient. Although it may seem obvious, one cannot stress enough that the most effective protection against irradiation of the hands is to remove them from the operating field and hide them behind the shields whenever fluoroscopy is used. When feasible, using the hands in the operating field and the foot on the fluoroscopy pedal alternately, so as to perform some maneuvers blindly and then check interval changes under fluoroscopy between each step, may be helpful to reduce hand exposure. For example, advancing a sheath over a wire in an easy case often does not require continuous use of fluoroscopy. However, such hand-foot alternation and coordination is not intuitive and requires some training (as commonly observed among interventional radiologists in training). In addition, it may not be feasible every time. For example, angiographers may want to perform hand injection of contrast under fluoroscopy, or may need to hold a catheter or wire because it tends to exit spontaneously from a percutaneous biliary or nephrostomy access site. In such cases, one strategy for the operator is to work with his or her arms around the ceiling shield: although the hands remain exposed, the rest of the body stays hidden behind the shield. Lastly, the patient-to-operator distance should be maximized whenever possible.
The findings of this study must be placed in perspective with other sources of scatter, and there are various parameters that the operator can modify to reduce the irradiation he or she receives. This investigation focused on (1) the SID and table height, (2) the table-suspended shielding, and (3) the ceiling-suspended shielding. Another important parameter, not explored here, is (4) the distance between the scatter source and the operator. If the scatter source were small, the inverse square distance law would apply; in the present experiment, where the scatter source was a large volume of phantom, distance provides less protection than expected by the inverse square of distance law. The present experiment chose to simulate percutaneous biliary or renal procedures as these are deemed potentially highly irradiating for the operator [8]. Interventions performed from a jugular, brachial or femoral approach would result in somewhat different values. Besides distance, other parameters modifiable by the operator to reduce his or her irradiation that were not studied here include:(5) the imaging field size or field of view, (6) patient thickness, (7) importance of an air gap, and (8) wearing personal protective apparel (lead apron, thyroid shield, and glasses).
One limitation of this experiment is that it focused on the primary operator and did not address the consequences to secondary operators and support staff; further research would be necessary to elucidate that question. Also, the phantom is a parallelepiped in shape, and the use of an anthropomorphic phantom might have shown different local variations in scatter. Furthermore, irradiation of angiographers is due not only to scatter from the phantom but also to leakage from the X-ray tube; however, the latter was negligible in the present experiment (<2%). Lastly, for simplicity we did not consider the case of oblique projections. Considerably higher scatter irradiation can be expected when operators are located close to the patient entrance surface and away from the flat panel detector (image intensifier).
In conclusion, the lead shields hanging at the tableside and from the ceiling of angiography suites provide very important protection against scatter from the patient to the operator and should be used whenever possible. Working at lower table heights provides additional protection to operators, although to a much lesser extent and at the cost of a higher entrance exposure rate to the patient. This incremental protection, although small in magnitude, could be substantial and clinically relevant in the long term as interventional radiologists use a relatively wide range of table heights during procedures and are exposed repeatedly for prolonged periods of time. Ultimately, the best table position is a compromise based as a minimum on patient exposure, scatter to operators, and operator comfort. Further work is warranted to describe more explicitly the tradeoff between these factors
References
Funama Y, Awai K, Umezu Y, Shimamura M, Ogawa K, Kato T, et al. (2005) Digital cine angiography permits radiation dose reduction without reduction in image quality. Radiat Med 23:151–155
Tsapaki V, Kottou S, Vano E, Komppa T, Padovani R, Dowling A, et al. (2004) Occupational dose constraints in interventional cardiology procedures: The DIMOND approach. Phys Med Biol 49:997–1005
Kuon E, Dahm JB, Empen K, Cole PE, Lu HT, Berenstein A, et al. (2004) Identification of less-irradiating tube angulations in invasive cardiology. J Am Coll Cardiol 44:1420–1428
Wagner LK, Archer BR, Cohen AM (2000) Management of patient skin dose in fluoroscopically guided interventional procedures. J Vasc Interv Radiol 11:25–33
Nikolic B, Spies JB, Campbell L, Walsh SM, Abbara S, Lundsten MJ (2001) Uterine artery embolization: Reduced radiation with refined technique. J Vasc Interv Radiol 12:39–44
Irie T (2004) Realtime digital magnification of the fluoroscopic and digital subtraction angiography images: randomized prospective study to show dose reduction during segmental chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol 15:165–168
Boone JM, Levin DC (1991) Radiation exposure to angiographers under different fluoroscopic imaging conditions. Radiology 180:861–865
Marx MV, Niklason L, Mauger EA (1992) Occupational radiation exposure to interventional radiologists: A prospective study. J Vasc Interv Radiol 3:597–606
Ito H, Hosoya T, Eguchi Y, Adachi M, Watanabe Y, Yamaguchi K (1999) Analysis of radiation scatter during angiographic procedures: Evaluation of a phantom model and a modified radiation protection system. J Vasc Interv Radiol 10:1343–1350
Irie T, Kajitani M, Itai Y (2001) CT fluoroscopy-guided intervention: Marked redaction of scattered radiation dose to the physician’s hand by use of a lead plate and an improved I-I device. J Vasc Interv Radiol 12:1417–1421
Haku T, Hosoya T, Ito H, Eguchi Y, Watanabe Y, Nishina M (2002) Radiation protection system for interventional procedures of the upper extremity: Evaluation in a phantom model. J Vasc Interv Radiol 13:815–822
Bush WH, Jones D, Brannen GE (1988) Radiation dose to personnel during percutaneous renal calculus removal. AJR Am J Roentgenol 145:1261–1264
Luchs JS, Rosioreanu A, Gregorius D, Venkataramanan N, Koehler V, Ortiz AO (2005) Radiation safety during spine interventions. J Vasc Interv Radiol 16:107–111
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s00270-007-9181-8.
Rights and permissions
About this article
Cite this article
d’Othée, B.J., Lin, PJ.P. The Influence of Angiography Table Shields and Height on Patient and Angiographer Irradiation During Interventional Radiology Procedures. Cardiovasc Intervent Radiol 30, 448–454 (2007). https://doi.org/10.1007/s00270-006-0063-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-006-0063-2