Abstract
Purpose
This prospective pilot study was undertaken to evaluate the feasibility and effectiveness of using a radiation absorbing shield to reduce operator dose from scatter during lower limb endovascular procedures.
Materials and Methods
A commercially available bismuth shield system (RADPAD) was used. Sixty consecutive patients undergoing lower limb angioplasty were included. Thirty procedures were performed without the RADPAD (control group) and thirty with the RADPAD (study group). Two separate methods were used to measure dose to a single operator. Thermoluminescent dosimeter (TLD) badges were used to measure hand, eye, and unshielded body dose. A direct dosimeter with digital readout was also used to measure eye and unshielded body dose. To allow for variation between control and study groups, dose per unit time was calculated.
Results
TLD results demonstrated a significant reduction in median body dose per unit time for the study group compared with controls (p = 0.001), corresponding to a mean dose reduction rate of 65 %. Median eye and hand dose per unit time were also reduced in the study group compared with control group, however, this was not statistically significant (p = 0.081 for eye, p = 0.628 for hand). Direct dosimeter readings also showed statistically significant reduction in median unshielded body dose rate for the study group compared with controls (p = 0.037). Eye dose rate was reduced for the study group but this was not statistically significant (p = 0.142).
Conclusion
Initial results are encouraging. Use of the shield resulted in a statistically significant reduction in unshielded dose to the operator’s body. Measured dose to the eye and hand of operator were also reduced but did not reach statistical significance in this pilot study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During interventional radiology procedures, such as lower limb angioplasty, operators can potentially receive significant dose from scatter. As procedures increase in complexity they often increase in length, requiring staff in the interventional suite to be in close proximity to the patient and X-ray tube for prolonged periods of time [1]. The harmful effects of ionising radiation are well documented [2], and the occupational effective dose limits that exist for staff working with ionising radiation should really be considered the upper limit of acceptability [1]. It is therefore extremely important to make attempts to reduce the dose from scatter to which workers are exposed wherever possible, in keeping with the principle of As Low As Reasonably Achievable (ALARA). Ways in which this can be achieved include the wearing of personal protective shielding such as lead aprons and thyroid collars as standard, while lead glasses can reduce dose to the eyes and lens. However, there remain parts of the body which are difficult to shield, and which can receive a significant dose from scatter, such as the hands [3]. Recently, a number of studies have shown that using a radiation absorbing drape/shield during certain interventional procedures can significantly reduce dose to operators [1, 4–6]. This prospective pilot study was undertaken to evaluate the feasibility and effectiveness of using such a radiation absorbing shield in order to reduce operator’s dose from scatter during lower limb endovascular procedures.
Materials and Methods
The shield used is commercially available (RADPAD, Worldwide Innovations and Technologies Inc, KS, USA), and lead free, composed of a composite material, mainly bismuth. The shielding properties are certified by the manufacturer as 0.125 lead equivalent. A wide range of shield types are available from the manufacturer which vary in size and shape depending on the intervention to be performed. When positioned on the patient, the shield absorbs scattered radiation from the patient and therefore should reduce dose to staff from scatter.
This was a single centre prospective observational study. A total of 60 consecutive patients undergoing endovascular intervention for peripheral vascular disease were included. The first 30 procedures were performed without the RADPAD and dose to operator was measured (control group). Operator dose was then measured for the following 30 procedures with the RADPAD in place (study group). Recorded doses were for a single operator (AT), who was the primary operator for the procedures. Informed consent for study participation was obtained from each patient at the time of the procedure. The study was performed as part of a quality assurance program with a view to monitoring and reducing dose to staff in the interventional suite, and so local ethics committee approval was waived.
All procedures were performed using a standard C-arm type IR unit (GE Medical systems, Milwaukee, USA), and with usual attention to good radiological practise such as careful collimation. Standard shielding included a hanging lead curtain beneath the patient table. A movable leaded glass shield fixed to the ceiling was used for digital subtraction angiography (DSA) runs. Standard personal shielding including a lead apron and protective thyroid collar were worn by the operator. Patient and procedural details were recorded prospectively. Total fluoroscopy time in seconds and dose area product (DAP, cGycm2) were recorded for each procedure.
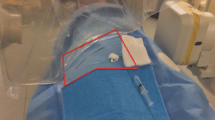
The radiation absorbing shields were placed on the patient prior to commencing the lower limb endovascular procedure. Two separate RADPAD shield types were used in conjunction for this study. The first was a peripheral shield (RADPAD # 5110, dimensions 11 × 34 in.), which was positioned alongside the patient’s leg nearest the operator, and covered the entire length of the lower limb (Fig. 1A). The second was a multipurpose interventional specialty shield (RADPAD # 5100, dimensions 12.5 × 16.5 in.), which was placed over the side of the patient’s chest nearest the operator (Fig. 1B). It has been shown by previous authors that patient skin entrance dose is not increased unless the protective shield is placed directly in the primary beam [1]. Every effort was therefore made to keep the shield out of the primary beam and any instance where the RADPAD became visible in the primary beam was documented.
A Peripheral radiation absorbing shield (RADPAD # 5110, dimensions 11 × 34 in.) for use in peripheral angiography. The shield was positioned on the side of the patient’s leg nearest the operator and covered the entire length of the lower limb. B Multipurpose interventional specialty radiation absorbing shield (RADPAD # 5100, dimensions 12.5 × 16.5 in.). The shield was placed over the side of the patient’s chest nearest the operator
To measure dose from scatter, the operator had a personalised set of thermoluminescent dosimeter (TLD) badges. To measure partial-body dose, a TLD was worn on the index finger of the left hand (dose to hand) and attached to the outside of the left side arm of the operator’s glasses (dose to eye). It has been previously shown that the left side of the operator is more exposed to scatter radiation in the interventional setting [3]. The unshielded personal dose (dose to body) was determined using a TLD fixed to the chest outside the operator’s lead apron. Similar methods for dose measurement have been employed in other studies [1, 3]. A separate set of TLDs were used for the control and study group. Dose measurements (mSv) were cumulative for each group. To account for differences in total fluoroscopy time and DAP between the two groups, a time-adjusted dose rate was calculated by dividing total measured dose by total fluoroscopy time for each of the two groups (dose per second fluoroscopy time, mSv/s). The significance of differences in the median dose levels per unit time was then tested by applying the Mann–Whitney rank sum test.
As a second additional method of measurement, a direct dosimeter (Unfors Instruments, Billdal, Sweden) with digital readout was used in some randomly selected cases, placed either on the left side arm of the glasses (14 cases), or at chest level outside the operators lead apron (12 cases). Cases were evenly distributed between the control and study groups. To allow comparison between groups, a time-adjusted dose rate was again calculated, by dividing measured dose by fluoroscopy time for each procedure (µGy/s). Mann–Whitney rank sum test was used to assess for differences in median dose per unit time between the control and study groups. Statistical analysis was performed using the SPSS Package.
Results
Patient demographics and procedure details are outlined in Table 1 and Table 2. The two patient groups showed similar characteristics, with no significant difference in age or body mass index between the groups. There was no significant difference in median fluoroscopy time or median dose area product in the study group compared with the control group. Eleven cases in the control group and 9 cases in the study group did not proceed to angioplasty or stenting and only angiography was performed. This occurred for a variety of reasons, such as the presence of diffuse disease or absence of significant stenosis. In the study group, the RADPAD appeared in the primary beam during 9 of the 30 cases (30 %). This was remedied through either repositioning (n = 4) or further collimation (n = 5).
The results from the TLD readings are outlined in Table 3. The TLD results show a statistically significant reduction in median body dose per unit time for the study group compared with the control group (p = 0.001). This corresponds to a median reduction of 65 % in the measured dose rate to the operator when the RADPAD was used. Median eye dose was also reduced in the study group but this was not statistically significant (p = 0.081). Although the cumulative hand dose appeared increased, median fluoroscopy time was longer for the study group, and when time-adjusted dose is calculated the dose to hand was also reduced in the study group compared with the control (3.409 × 10−4 mSv/s vs. 4.012 × 10−4 mSv/s). This was not statistically significant, however (p = 0.628).
The TLD data were supported by the direct dosimeter readings (Table 4). Direct dosimeter readings showed a reduction in median unshielded body dose rate for the study group (3.01 × 10−2 µGy/s) compared with the control group (5.91 × 10−2 µGy/s). This was statistically significant (p = 0.037) and corresponded to a median reduction of 49 % in the measured unshielded body dose rate. Median dosimeter readings for the eye also showed a reduction in the study group, however this was not statistically significant (p = 0.142).
Discussion
The increasing scope and complexity of interventional fluoroscopic procedures have increased both patient and staff ionising radiation doses. In addition, there has been a reduction in operator dose limits presenting additional radiation protection challenges.
The dose limits to the ocular lens was previously 150 mSv/year as it was believed that the threshold dose for cataract formation was 2 Gy for acute exposure and under 5 Gy for prolonged exposure. The International Commission on Radiation Protection (ICRP) have revised the lens dose limits to 20 mSv/year averaged over 5 years (100 mSv in 5 years) with no single year exceeding 50 mSv for occupational exposure in planned situations. This is in recognition of recent studies suggesting that there may not be a threshold dose for cataract formation and cataracts may be induced at doses lower than 2 Gy [7, 8]. ICRP now recommend 500 mGy as a threshold dose for practical purposes [9], although it has been suggested that radiation cataractogenesis may follow a linear, no-threshold model [10].
Radiation is also the only unequivocal risk factor for glial and meningeal neoplasms [11]. There are case series of interventionalists with left hemisphere brain tumours, the side that gets the most radiation [12, 13]. An increased risk of neck tumours have also been demonstrated [14].
A number of studies have previously shown that using a radiation absorbing shield during certain interventional procedures can significantly reduce dose to operators [1, 4–6]. King et al. demonstrated the use of such a device during percutaneous nephrostomy procedures and showed a significant reduction in scatter to the eyes (12-fold reduction), thyroid (25-fold reduction) and hands (29-fold reduction) of operators [1]. Other investigators report reduction in dose from scatter during pectoral device implantation [4, 5], electrophysiology procedures [4], as well as abdominal [6] and cardiac angiography procedures [6, 15]. We report similar findings, showing a reduction in the measured dose to body, eye and hand when using such a radiation absorbing shield. Our findings were corroborated using two different dose measurement systems, with similar results. However, in our study, only the measured dose reduction to body was found to be statistically significant. This may relate to a number of factors. For procedures such as pectoral device implantation, the operator is typically in closer proximity to the image intensifier field of view than would usually be the case for lower limb endovascular procedures and therefore overall dose reduction benefits of the RADPAD may be greater for such close proximity procedures. Also we included a relatively small number of patients in this pilot study and it is possible that with increased numbers dose reduction for the eye and hand would also reach statistical significance.
Similar to other authors [1, 5, 15] we positioned dosimeters outside any personal protective equipment, i.e. on the outside of the sidearm of the operator’s glasses and outside the lead apron. We did not place a dosimeter under the operator’s lead apron to measure the protected body dose. In the interventional radiology setting, the wearing of a dosimeter under a lead apron is intended to provide a measurement which is closely related to the effective dose [16, 17]. There is however a controversy as to whether this provides a realistic assessment of the effective dose as the dosimeters worn by the majority of radiology staff either never register a reading or register a low dose which is close to the recording threshold [17]. The data from an under the lead dosimeter would therefore not have been sufficiently reliable to allow assessment of dose reduction for our study. Many countries use either a single dosimeter outside at the collar or a two-dosimeter approach (under the lead apron and outside at the collar) for monitoring of exposure to staff in an interventional setting. Using a single dosimeter outside personal protective equipment can give an indication of the dose level in the interventional suite and record a dose which is sufficiently large to allow a strategy for dose monitoring [17] and therefore an assessment of dose reduction. It was this rationale that led us to place dosimeters outside personal protective equipment for our study.
It has been shown by previous authors that patient skin entrance dose is not increased unless the shield is placed directly in the primary beam [1]. We made every effort to avoid entry of the RADPAD to the primary beam however, entry still occurred in 30 % of procedures. In 5 of the 9 cases, this could be remedied by further collimation; however, repositioning was required in 4 cases. Requirement for repositioning of the shield can be inconvenient particularly in technically challenging procedures and if a recurrent issue could potentially add to the overall procedure time and dose to patient.
Our study does have some limitations, including, as discussed a relatively small number of study participants. However, it was undertaken as a pilot study to assess feasibility of using such a radiation absorbing shield for lower limb endovascular procedures, something which has not previously been reported. It was a single centre and single operator study. However, using only a single operator allowed for consistency throughout the study. The study was not randomized, rather consecutive patients were enrolled first for the control group and subsequently for the study group; however, patient demographics did not differ significantly between the groups. Although it is possible that operator experience was greater for the second i.e. study group, overall fluoroscopy times for this group were actually longer. Differences in procedure complexity and fluoroscopy were accounted for using a time-adjusted dose rate, allowing inter-group comparisons.
Conclusion
In summary, initial results from this pilot study are encouraging and suggest that a radiation absorbing shield can reduce operator dose from scatter during lower limb endovascular procedures. Use of the shield resulted in a statistically significant reduction in unshielded dose to the operator’s body, corresponding to a 49–65 % median dose rate reduction. Measured dose to the eye and hand of the operator was also reduced but reduction did not reach statistical significance in this pilot study. The shield was easy to use, and although it was seen to enter the primary beam, this could in the majority be managed by further collimation.
References
King JN, Champlin AM, Kelsey CA, Tripp DA (2002) Using a sterile disposable protective surgical drape for reduction of radiation exposure to interventionalists. AJR Am J Roentgenol 178:153–157
Amis ES, Butler PF, Applegate K et al (2007) American college of radiology white paper on radiation dose in medicine. J Am Coll Radiol 4:272–284
Hausler U, Czarwinski R, Brix G (2009) Radiation exposure of medical staff from interventional X-ray procedures: a multicentre study. Eur Radiol 19:2000–2008
Germano JJ, Day G, Gregorious D, Natarajan V, Cohen T (2005) A novel radiation protection drape reduces radiation exposure during fluoroscopy-guided electrophysiology procedures. J Invasive Cardiol 17:469–472
Simons GR, Orrison WW (2004) Use of a sterile, disposable, radiation-absorbing shield reduces occupational exposure to scatter radiation during pectoral device implantation. Pacing Clin Electrophysiol 27:726–729
Lida H, Horii J, Chabatake M, Mizushima T (2004) Evolution of radiation exposure to operator in diagnostic and interventional radiology procedures and reduction of radiation exposure to operator with protective devices (Abstract Only). Nippon Hoshasen Gijutsu Gakkai Zasshi 60:1713–1722
Nakashima E, Neriishi K, Minamoto A (2006) A reanalysis of atomic-bomb cataract data, 2000–2002: a threshold analysis. Health Phys 90:154–160
Neriishi K, Nakashima E, Minamoto A et al (2007) Postoperative cataract cases among atomic bomb survivors: radiation dose response and threshold. Radiat Res 168:404–408
Authors on behalf of ICRP, Stewart FA, Akleyev AV, Hauer-Jensen M et al (2012) ICRP publication 118: ICRP statement on tissue reactions/early and late effects of radiation in normal tissues and organs—threshold doses for tissue reactions in a radiation protection context. Ann ICRP 41:1–322
Ainsbury EA, Bouffler SD, Dörr W et al (2009) Radiation cataractogenesis: a review of recent studies. Radiat Res 172:1–9
DeAngelis LM (2001) Brain tumors. N Engl J Med 344:114–123
Roguin A, Goldstein J, Bar O (2012) Brain tumours among interventional cardiologists: a cause for alarm? Report of four new cases from two cities and a review of the literature. EuroIntervention 7:1081–1086
Roguin A (2012) Brain tumours among interventional cardiologists: a call for alarm? Eur Heart J 33:1850–1851
Roguin A, Goldstein J, Bar O, Goldstein JA (2013) Brain and neck tumors among physicians performing interventional procedures. Am J Cardiol 111:1368–1372
Politi L, Biondi-Zoccai G, Nocetti L et al (2012) Reduction of scatter radiation during transradial percutaneous coronary angiography: A randomized trial using a lead free radiation shield. Catheter Cardiovasc Interv 79:97–102
Miller D, Vañó E, Bartal G et al (2010) Occupational radiation protection in interventional radiology: A joint guideline of the Cardiovascular and Interventional Radiology Society of Europe and the Society of Interventional Radiology. Cardiovasc Intervent Radiol 33:230–239
Martin CJ (2012) Personnel dosimetry in UK radiology: is it time for a change? J Radiol Prot 32:E3–E6
Conflict of interest
Michael Lee, Sarah Power, Mahmood Mirza, Ajay Thakorlal, Bhaskar Ganai, Linda Gavagan and Mark Given declares no conflict of interest.
Statement of Informed Consent
Informed consent was obtained from all individual participants included in the study.
Statement of Human Rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Power, S., Mirza, M., Thakorlal, A. et al. Efficacy of a Radiation Absorbing Shield in Reducing Dose to the Interventionalist During Peripheral Endovascular Procedures: A Single Centre Pilot Study. Cardiovasc Intervent Radiol 38, 573–578 (2015). https://doi.org/10.1007/s00270-014-0997-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-014-0997-8