Abstract
A pulmonary arteriovenous fistula (PAVF) is a rare vascular malformation commonly treated by embolization with coils or balloons to prevent the risk of several serious complications such as cerebral embolism and brain abscess. A 32-year-old female with two PAVFs and neurological ischemic manifestations has been successfully treated by transcatheter embolization of both fistulas using a new device (Amplatzer Vascular Plug). This self-expanding cylindrical nitinol mesh cage with high radial strength allows a chance of relocation until properly positioned. It is preferred to coils or balloons because a large caliber of feeding artery implied high risk of uncontrollable distal embolization. There appear to be no reports in the literature concerning use of this device, which could represent a useful innovative tool in embolotherapies, especially in large vascular areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Pulmonary arteriovenous fistula (PAVF), a rare vascular abnormality, is a direct communication between the pulmonary artery and vein without an intervening capillary bed, thus representing a right-to-left cardiac shunt. Common symptoms are exertional dyspnea, fatigability, palpitations, and cyanosis. Severe complications, such as cerebral embolism with brain abscess, transient ischemic attack, or stroke have been reported [1–3].
These fistulas are often associated with hereditary hemorrhagic telangiectasia, or Rendu-Osler-Weber disease (ROWD).
The primary aim of any treatment is to eliminate or reduce right-to-left shunt and to prevent complications and, indeed, effective treatment is possible, using transcatheter techniques or surgery [1–3]. Although transcatheter embolization, with coils or occlusion detachable balloons, is considered the treatment of choice [2, 4, 5] technical difficulties, such as air embolism or paradoxical device embolization and recanalization, have been reported [5, 6]. A case of succesful PAVF embolization using the Cardioseal (NMT, Boston, MA) umbrella device [7] and recently a case using the TriSpan Coil (Boston Scientific) to prevent distal migration have been reported in the literature [8].
A case is described here of a large PAVF successfully treated using a new embolization device, the Amplatzer Vascular Plug, produced by Amplatzer Medical. To our knowledge this is the first report in the literature describing use of the new device in this type of fistula.
Case Report
A 32-year-old woman was referred, in another hospital, because of lumbalgia and weakness of the left lower limb. Preliminary clinical diagnosis was herniated disk (L4–L5). After magnetic resonance imaging (MRI) confirmation and nonresponse to medical treatment, the patient underwent microdiscectomy. Lumbalgia regressed but weakness persisted. Five days later, the patient complained of paresthesia and dysesthesia of the trunk and right upper and lower limbs. The patient was then admitted to our center and was followed in the Clinical Neurology Unit. A brain T2-weighted MRI scan executed by our group 3 days after admittance showed an ischemic lesion in the left hemisphere. The young age of the patient prompted the need for general investigations, and molecular genetic analysis revealed the presence of a mutation on the ALK-1 gene, frequently associated with ROWD. Hemoglobin concentration was 10.4 g/dl, red blood cell count was 4.4 × 1012 cells per liter, and arterial oxygen saturation was 76%.
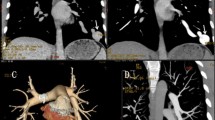
Transthoracic and transesophageal echocardiography before and after contrast media (Xenetix Iobitridolo 350) injected into an arm vein showed opacification of both the right and left heart, indicating right-to-left shunt. A plain chest film showed two nodular lesions, in the right pulmonary middle lobe (max diameter 1 cm) and left lower lobe (3 cm); a 16-slice spiral computed tomography (CT) scan (Philips MDT MX 8000) demonstrated marked contrast media enhancement. Multiplanar reconstruction (MPR) and Volume Rendering reconstructions (GE Advanced Vessel Analysis work station) then accurately revealed the feeding arteries and draining veins for each lesion, suggesting PAVFs (Fig. 1).
Written consent was obtained and an endovascular procedure was planned to treat, first of all, the larger lesion.
Right transfemoral venous access was chosen, and left pulmonarv artery catheterization and multiple angiograms were performed, Heparin (2500 U) was given in bolus after the femoral approach and another 2500 U in saline was injected during the procedure with continuous flushing of the guiding catheter to prevent pericatheter thrombosis. Selective catheterization of the feeding artery of the major fistula with a 6F multipurpose guiding catheter (mach one, Boston Scientific) and hydrophilic guide wire (Terumo), A-P and oblique views were taken showing a high-flow large fistula and rapid opacification of the draining vein (Fig. 2). The diameter and length of the feeding artery were calibrated and measured. The artery was 6 mm in diameter. Considering the relatively large diameter of the fistula and flow rate, it was decided to proceed with embolization using the Amplatzer Vascular Plug. This is a newly developed self-expandable, cylindrical embolization device with high radial strength, made from a highly flexible Nitinol wire mesh secured at both ends with platinum/iridium marker bands, making it more visible under fluoroscopy (Fig. 3). The shape of the plug is very simple, not unlike a cage (Fig. 4). A stainless steel microscrew is welded to one of the platinum/iridium marker bands, allowing attachment to the 135-cm-long stainless steel delivery cable. The delivery system allows the operator to check whether the device is in the appropriate position and whether it is stable, under blood pressure, before starting the detachment maneuver. Soon after deployment, the Nitinol mesh induces progressive local thrombosis that completes in a few minutes. In the case of incomplete thrombosis, further deployment of coils could be safely performed.
The large diameter of the feeding artery and the high-flow pattern of the fistula gave rise to some concern regarding possible distal migration of traditional embolization devices. Another reason for selecting this device was the fact that it is designed for embolotherapy in the peripheral vasculature, with a wide range of different sizes, to fit a variety of vessels. According to the manufacturer’s recommendations, the Vascular Plug should exceed the diameter of the vessel to be embolized by 30–50%.
The diameter and length of the selected device (in the unconstrained status) were 10 mm and 7 mm, respectively.
Once preloaded and adequately flushed to remove air from the loader, the plug was advanced along the guiding catheter as far as the end, inside the distal part of the feeding artery. The catheter was then pulled backwards allowing the plug to expand. A test injection through the catheter confirmed the correct position of the Vascular Plug, which was then released by rotating the delivery cable in a counterclockwise fashion (Fig. 3). Unscrewing the supporting wire from the Vascular Plug was easily and rapidly achieved, and the device did not move during this maneuver. Thrombosis was completed within approximately 5 min.
Immediately after delivery, a marked reduction of flow was observed in the fistula, but the patient complained of angor and tension on the left side of the body. ST segment depression, on the electrocardiogram (ECG), confirmed a cardiac ischemic impairment; blood pressure was 80/40 mmHg. Appropriate medical treatment was commenced immediately, with oxygen 100%, morphine 3 mg, nitroglycerine 5 mg, and midazolam 2 mg. Regression of symptoms and return to normal blood pressure and ECG occurred within 15 min. Five minutes after the delivery, angiography confirmed the correct position of the device as well as complete embolization of the AV malformation (Fig. 3). Arterial oxygen saturation rose to 90%.
After this procedure, the patient was completely asymptomatic, albeit, as a precaution, she was transferred to the intensive care unit (ICU) for 24 hr. Analysis of myocardial enzymes, tested in the ICU, revealed increased values only of troponine I (0.8 ng/ml), thus confirming myocardial impairment. Over the next few days, the patient remained asymptomatic. The troponine I value decreased to 0.4 ng/ml on the second day, and to 0.3 on the third. Modifications observed in the repolarization phase improved within a few days. Following the advice of the cardiologist, embolization of the other smaller fistula was postponed. The patient was discharged 2 days after the embolization. Three weeks later the patient was readmitted. A control 16-slice CT scan showed complete thrombosis of the previously treated fistula, which was also reduced in diameter (Fig. 4). The smaller fistula was treated by the use of the same technique. The procedure was successfully completed, without any kind of complication, using a 4-mm-large and 7-mm-long Vascular Plug (Fig. 5).
Discussion
The treatment of choice for patients with significant PAVF is embolotherapy with coils or detachable occlusion balloons [2, 4, 5]. However, some PAVFs may give rise to technical problems and be difficult to occlude. Potential complications, such as asymptomatic air embolism, paradoxical embolization of a device, self-limited pleurisy and angina secondary to air embolus, as well as persistence of the fistula, due either to recanalization or interim accessory artery growth, have been reported [5, 6, 9]. However, successful embolotherapy of large PAVFs, using standard devices and detachable coils, has been reported, even if in some cases two or more embolizations, in different sessions, were needed [5, 10].
Coil embolotherapy requires accurate selection of coil diameters. The coils, once delivered, should spontaneously assume their shape and adhere to the vessel wall. Because of high flow rate and unpredictable wall elasticity, overestimation of the coil size is mandatory. However, accidental coil migration may occur, arising from the aorta, with severe consequences. Detachable coils of large diameter are not available on the market because their main field of application is in neurovascular intervention. Occlusion balloons could be used in these fistulas, but early balloon deflation or elastic vascular wall remodeling can, although rarely, cause balloon migration [11].
The innovation in our procedure lies in the use of the Amplatzer Vascular Plug. The precursors of this device are the Amplatzer Septal Occluder and the Amplatzer Duct Occluder used in the treatment of atrial septal defects and patent ductus arteriosus, respectively. Several reports in the literature have described successful transcatheter treatment of congenital malformations with these Nitinol devices [12, 13]. Their shapes reflect the uses for which they are intended, but the flexible quality of the Nitinol together with the different designs of those occluders make it possible for these plug devices to be used for conditions other than what they are designed for, and they have been used to occlude pulmonary arteriovenous malformation and to close an aorto-left ventricular tunnel [14].
The Vascular Plug has a simple shape and the same Nitinol structure of its precursors. The reported risk of paradoxical embolization of coils or balloons during transcatheter occlusion of PAVFs ranges from 2% to 4%. The incidence of persistence or recurrence of the fistulas has been reported to be around 16% [5, 9].
In our opinion, use of the Vascular Plug can significantly reduce the risk of paradoxical embolization because the size and shape of the device provides radial strength and stable adhesion to the vessel walls.
Bearing in mind the efficacy of the Amplatzer Septal Occluder and Duct Occluder in the treatment of the defects for which they were developed [12, 13], the risk, in our opinion, of persistence or recurrence of the fistula, due to recanalization, should be low with the use of this new, but similar, device. Long-term results, however, are not yet avaliable. Our procedure was successful, the delivery was precise, and embolization was perfectly achieved. The reported transient complication appears to be caused by an intense vasovagal reflex with drop of the blood pressure, reduction of the coronary flow, and consequent myocardial temporary ischemia. Another explanation is a coronary air embolism caused by inadequate flushing of the delivery apparatus, already reported as a possible rare complication in the literature [5]. Clot formation on the device and distal embolization should be also evaluated, but considering the short time elapsed between the deployment of the device and the onset of symptoms, this hypothesis seem to be less likely. However, although it is certainly related to the procedure, there is no evidence of any direct relationship between the complication and the type of device used.
The Amplatzer Vascular Plug appears to be an effective tool for embolotherapy of PAVFs, particularly in cases of large feeding and draining vessels, at increased risk of paradoxical embolization when using coils or detachable occlusion balloons. According to our experience considering the shape, the different sizes available, and the flexible quality of the material, this device has promising features as one of the most effective tools in peripheral arterial embolization. However, information on the use of other available sizes and on long-term results should be supplied by future investigations.
References
Pick A, Deschamps C, Stanson AW (1999) Pulmonary arteriovenous fistula: presentation, diagnosis, and treatment. World J Surg 23:1118–1122
White RI, Pollack JS, Wirth JA (1996) Pulmonary arteriovenous malformations: diagnosis and transcatheter embolotherapy. J Vase Interv Radiol 7:787–804
Swanson KL, Prakash UB, Stanson AW (1999) Pulmonary arteriovenous fistulas: Mayo Clinic experience, 1982–l997. Mayo Clin Proc 74:671–680
White RI Jr, Lynch-Nyhan A, Terry P, et al. (1988) Pulmonary arteriovenous malformations: techniques and long-term outcome of embolotherapy. Radiology 169:663–669
Lee DW, White RI Jr, Egglin TK, et al. (1997) Embolotherapy of large pulmonary arteriovenous malformations: long-term results. Ann Thorac Surg 64:930–993 discussion 939–940
Sagara K, Miyazono N, Inoue H, et al. (1998) Recanalization after coil embolotherapy of pulmonary arteriovenous malformations: study of long-term outcome and mechanism for recanalization. AJR Am J Roentgenol 1701:727–730
Apostolopoulou SC, Kelekis NL, Papagiannis J, et al. (2001) Transcatheter occlusion of a large pulmonary arteriovenous malformation with use of a Cardioseal device. J Vase Interv Radiol 12:767–769
Cil BE, Erdogan C, Akmangit I, et al. (2004) Use of the TriSpan coil to facilitate the transcatheter occlusion of pulmonary arteriovenous malformation. Cardiovasc Intervent Radiol 27:655–658
Lacombe P, Lagrange C, El Hajjam M, et al. (2005) Reperfusion of complex pulmonary arteriovenous malformations after embolization: report of three cases. Cardiovasc Intervent Radiol., 28:30–35
Takahashi K, Tanimura K, Honda M, et al. (1999) Venous sac embolization of pulmonary arteriovenous malformation: preliminary experience using interlocking detachable coils. Cardiovasc Intervent Radiol 22:210–213
Saluja S, Sitko I, Lee DW, et al. (1999) Embolotherapy of pulmonary arteriovenous malformations with detachable balloons: long-term durability and efficacy. J Vase Interv Radiol 10:883–889
Wang JK, Tsai SK, Wu MH, et al. (2004) Short- and intermediate-term results of transcatheter closure of atrial septal defect with the Amplatzer Septal Occluder. Am Heart J 148:511–517
Santoro G, Bigazzi MC, Palladino MT, et al. (2004) Comparison of percutaneous closure of large patent ductus arteriosus bv multiple coils versus the Amplatzer duct occluder device. Am J Cardiol 94:252–255
De Giovanni JV (2001) The use of Amplatzer devices to occlude vascular fistulae. J Interventi Cardiol 14:45–48
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rossi, M., Rebonato, A., Greco, L. et al. A New Device for Vascular Embolization: Report on Case of Two Pulmonary Arteriovenous Fistulas Embolization Using the Amplatzer Vascular Plug. Cardiovasc Intervent Radiol 29, 902–906 (2006). https://doi.org/10.1007/s00270-005-0160-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-005-0160-7