Abstract
A natural orange diamond crystal from Siberian kimberlite has been studied using complementary spectroscopic techniques. The IR-spectra of the crystal revealed presence of low concentration of nitrogen A, B and C centers. In addition to these common defects a Y-center and 1240 cm−1 band were clearly detected. Absorption UV–Vis spectra exhibited the 490 nm band and an unknown very broad band with diffuse maximum at 320–380 nm, with low energy edge extending far into the visible region. Photoluminescence spectra showed presence of N3, 440 nm, 455 nm, S1, 575.8 nm vibronic systems as well as zero-phonon lines at 418.2, 525.1, 560.8, 645.7 and 799.7 nm. Luminescence of the 575.8 nm system with broad vibronic band peaked at 690 nm noticeably contributes to the color of the diamond, i.e. its orange color is not only due to absorption but also to emission of light. Electron Paramagnetic Resonance revealed several structural defects: nitrogen-related P1, M5, M6 centers, oxygen-related OK1 and N3 centers, previously unreported nickel M7 center and tentatively phosphorus-related W24 center. The M5 and M6 centers involving two nitrogen atoms separated by ca. 8–10 interatomic distances represent an unusual long-range bonding. It is suggested that M6 and M7 might be related to the IR Y-center and to the broad band with maxima at 320–380 nm in the optical spectra of the diamond.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cause of valuable orange coloration of natural diamond remains poorly understood yet. Pure orange diamonds are encountered extremely rarely in nature though orange crystals with a yellow or brown hue are more common (Hofer 1998). One of rare described sources of orange diamonds is kimberlite (Matsyuk et al. 1990) and placer (Zudina et al. 2013) deposits of Siberia.
It is assumed that the orange coloration of pure spectral tone in diamonds is connected with a band in spectra of absorption with a maximum around 480 nm, underlined by absorption continuum starting at ~500 nm and increasing considerably towards shorter wavelengths (Collins and Mohammed 1982; Fritsch 1998; Collins 2001). The absorption continuum is assigned to single substitutional nitrogen atoms—the C-defects (Collins 2001; Zaitsev 2001). The 480 nm band is ascribed to O+ defect (Gali et al. 2001). Some orange diamonds show only the absorption continuum shifted towards long-wave region of the spectra and some absorbance from 980 nm onwards (Hainschwang et al. 2013).
The present work is devoted to study of point defects in an orange diamond crystal from kimberlites of the Siberian platform for revealing the nature of its coloration. The crystal attracted our attention by slight changes of the coloration with illumination. At the same time its color tone was rather light, in contrast to the majority of orange diamonds investigated previously, being favorable for detailed optical studies. This diamond has been studied using a set of spectroscopic methods: UV–Vis absorption spectroscopy, IR-spectroscopy, photoluminescence and EPR-spectroscopy.
The sample and methods
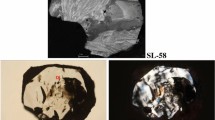
The studied sample was a pear-shaped brilliant-cut diamond 1.07 ct (0.214 g) in weight. According to the grading system of the Gemological Institute of America its color may be described as Fancy Vivid orangy-Yellow under incandescent light, Fancy Vivid yellowish-Orange under gemological lamp (Fig. 1a) and as Fancy Vivid Orange under bright sky light (Fig. 1b). In all the cases its color was surprisingly attractive. The clarity grades of the diamond studied fell into the I2 (included) category.
Before cutting the diamond possessed a cubic habit, was 3.5 ct in weight. It was mined from a kimberlite pipe of the Siberian platform. The crystal exhibited nearly flat faces with numerous tetragon etch pits. It had a light grey coat overgrowing yellow-orange core. In rough the studied diamond corresponds to the III variety of mineralogical classification of diamonds by Orlov (1977) while its inner part revealed at cutting, to the II variety.
Infra-red spectra were obtained using the diffuse scattering attachment to FTIR Perkin Elmer System-2000 spectrometer in 400–6000 cm−1 spectral range at 4 cm−1 resolution. Spectral decomposition was performed by trial-and-error method of combination of components due to pure A, B, C defects taken from the software developed by D. Fisher (DTC, Maidenhead). Concentrations of the main nitrogen defects were determined after standard normalization for lattice absorption and using calibration coefficients proposed by Boyd et al. (1994, 1995). We note that for diamonds with pronounced contribution of the C-defects possessing a narrow peak at 1344 cm−1 the spectra recorded at 4 cm−1 resolution tend to underestimate content of theses defects, but the effect is not very strong.
The UV–Vis absorption spectra were measured with a double-beam Perkin-Elmer Lambda-9 spectrophotometer in transmission and reflectance geometries at room temperature.
The photoluminescence (PL) and photoluminescence excitation (PLE) spectra were recorded with HORIBA Jobin–Yvon Fluorolog-FL3-221 spectrometer at room (ca 295 K) and liquid nitrogen (77 K) temperatures. PL spectra were recorded under excitation with the wavelengths in the range of 250–650 nm. PLE spectra were registered in the range of 350–750 nm. Slit width was 0.5–1 nm. The PL and PLE spectra were corrected for detector spectral sensitivity and on the xenon lamp spectral distribution. For precise determination of position of lines of some new centers additional calibration of the spectrometer was performed. The monochromator wavelengths scale was calibrated using the standard Hg lamp, and excitation monochromator was calibrated using dedicated absorption filter (TAS-1). An error in the determination of the spectral lines positions was ±0.3 nm.
EPR-spectra were recorded using a Varian E-115 X-band spectrometer with modulation frequencies of 100 and 25 kHz at room temperature. The magnetic field was measured on an E-500 NMR gauss meter. To analyze the peculiarities of spectra along different crystal directions, the sample was rotated in the spectrometer using a biaxial goniometer. The accuracy of orientation of the crystal in respect to the applied magnetic field is about 0.5°. The directions of the axes of the diamond specimen were determined using the spectra of the P1 paramagnetic center, whose dependence on crystal directions is well established. The concentrations of paramagnetic centers in the diamonds were determined by comparing the spectral line intensities of a sample studied with a standard DPPH sample with known amount of paramagnetic centers (2.61 ppb).
IR-spectroscopy
In IR-spectra of the orange diamond a large number of structural defects were revealed. According to spectral decomposition (Fig. 2), the studied crystal contains all three principal nitrogen defects A (two N atoms in the nearest structural positions, see review by Zaitsev (2001) for models of defects in diamond), B (4 N atoms around a vacancy) and C (single nitrogen atom) simultaneously. The concentration of these defects is ca. 10 ppm, 10 ppm and 15 ppm, respectively. The presence of the B-defect is supported by a clear absorption feature at 780 cm−1 (Zaitsev 2001). By total nitrogen content (ca. 35 ppm) the diamond fall into group 1, i.e. low-nitrogen crystals, according to IR diamond classification by (Kaminsky and Khachatryan 2001). Due to the low concentration of the A defects there is very little quenching of the luminescence in the sample. The diamonds with all main N-related defects are rarely encountered. According to the physical classification they fall into IaAB + Ib type. Similar diamonds with somewhat higher B defect content were described recently as “ABC diamonds” (Hainschwang et al. 2006). Simultaneous presence of all these defects, characterized by very different formation temperature, might be explained by internal zoning of the diamond studied with highly aggregated nitrogen in the core. Alternatively, the ABC diamonds might be produced by extreme overheating resulting in partial dissociation of A- and B-defects, which possibility was shown experimentally (see Dobrinets et al. 2013 for review).
Subtraction of combined contributions of A, C, and B defects from the experimental spectrum leaves a noticeable residue (Fig. 2). This residual absorption most likely represents overlapping Y-defect (principle band at 1145 cm−1) (Hainschwang et al. (2012) and a 1240 cm−1 band (Woods and Collins 1983), whose intensity are close to the intensity of the major nitrogen defects. These bands are rarely encountered in IR-spectra of natural diamonds in general. It is suggested that the center Y represents another, distinct from the C defects, form of single substitutional nitrogen atom in diamond structure (Hainschwang et al. 2012). The defect corresponding to the 1240 cm−1 band remains unknown.
The weak lines at 1353, 1358, 1363 and 1387 cm−1, detected in the IR-spectra of the orange crystal, have been revealed earlier in diamonds with Y defects and were ascribed to their local vibrations (Hainschwang et al. 2012). Rather weak lines at 1428, 1510, 1518 cm−1 were also observed. The peak at 1428 cm−1 may be due to carbonate microinclusions (e.g. Shiryaev et al. 2005).
Well-known hydrogen-related lines at 1405 and 3107 cm−1 are present in the IR spectrum (Woods and Collins 1983, Zaitsev 2001). In the range of 3000–3400 cm−1 a set of rather weak lines at 3120, 3138, 3145, 3156, 3182, 3200, 3227, 3235, 3257, 3270, 3298 cm−1 is observed (Fig. 3). Position of these lines coincide within ±1 cm−1 with presumably H-related features reported recently in diamonds with the Y defect (Hainschwang et al. 2012). The diamond studied is characterized by an unusually high intensity of a line at 3182 cm−1, considerably exceeding the intensity of a common hydrogen line at 3107 cm−1. The 3182 cm−1 line, as well as features at 3138 and 3145 cm−1, have been tentatively ascribed to the vibrations of the N–H bonds (Woods and Collins 1983; Zaitsev 2001).
A set of lines ascribed to hydrogen impurities (Hainschwang et al. 2012) in the IR-spectrum of the crystal. More intense lines are arrowed
Absorption spectroscopy
Due to low intensity of the coloration it was possible to record the absorption spectra of the diamond specimen down to 250 nm. In previous studies of similar crystals saturation occurred at wavelengths less than 390 nm due to higher nitrogen content (Collins and Mohammed 1982). The absorption spectra (Fig. 4) show a band peaked at about 490 nm and another very broad complicated band in the region of 320–380 nm. The long-wave edge of the broad band in the 320–380 nm region extends into the visible region of the spectrum and influence the color of the crystal. The absorption continuum starting at about 500 nm apparently represents a long-wave part of the band detected. Clearly, this band consists of several components, whose origin will be discussed below together with PLE spectra.
The band at 480 nm was previously revealed in natural brown or amber-brown diamonds (Collins and Mohammed 1982; Pereira et al. 1986; Pereira and Jorge 1987). The color of diamonds with the 490 nm band in the absorption spectra under standard illumination conditions, was described as orange (Hofer 1998; Fritsch 1998). This band is characteristic for CO2 containing diamonds (Hainschwang et al. 2008). The 490 nm band corresponds to the band at 690 nm in the PL (see below). PLE spectrum of this center is fully consistent with the absorption.
A broad band at 320–380 nm is rarely observed in natural diamonds. Similar bands were observed in brown-yellow (Wang and Moses 2003) and pink-purple diamonds (Titkov et al. 2008).
We note that the overall absorption spectrum of the diamond specimen (Fig. 4) rather corresponds to a crystal with a brownish-yellow coloration though in reality the color is orange. The origin of this controversy is described below.
Photoluminescence
The PL spectra of the orange diamond display a great variety of structural defects observed in the whole visible range (Fig. 5). The most intense is a vibronic band with a maximum at 690 nm which is produced by absorption in 490 nm band. The related zero-phonon line (ZPL), is weak and is not always observed (Collins and Mohammed 1982). Pereira et al. (1986) detected (at 23 K) twelve very weak lines assigned to zero-phonon transitions in the spectral region 572–604 nm, where the ZPL of the center should be observed. The 690 nm vibronic band was ascribed to a ZPL at 577.8 nm. It should be noted that earlier Plotnikova et al. (1980) have found a similar luminescent system with ZPL at 578 nm but the vibronic band shape was rather different.
Another feature of the center with 690 nm vibronic band is that in many cases the PLE band is unstructured, while the PL band shows a distinct vibronic structure. This is due to weakening of the electron–phonon coupling in the center upon conversion into excited state (Collins and Mohammed 1982). Only one study (Pereira and Jorge 1987) reports weak vibronic structure of the PLE band of this center. The phonon energy, determined by the vibronic structure of the PLE band was 23 meV, while the vibronic structure of the PL band indicates the coupling with 30 meV phonons. In our specimen, the 690 nm band exhibits very distinct and unusual vibronic progression: each phonon sideband is split into two components, separated by an interval of 10 meV at any wavelengths of the exciting light. All the first components of each phonon band corresponded to the coupling with ~30 meV phonons (separated from each other by ~30 meV). The splitting of the phonon sidebands corresponding to the coupling with ~34 meV phonons was also observed in the PLE spectrum, but it was much less pronounced.
In the region where the ZPL of the center may occur, a number of weak lines at 572.9, 575.8, 578.8, 582.3, 584.6, 587.7 nm were found. Comparison of the PL and PLE spectra (Fig. 6) showed that only the lines at 572.9 and 575.8 nm can be attributed to the zero-phonon transitions. The vibronic progression is probably due to the line at 575.8 nm (2.153 eV), since its separation from the first phonon sideband (or rather its first component) is 32 meV, which is close to the distance between the subsequent sidebands. In the PLE spectrum, the separation between 575.8 nm line and the first phonon sideband is ca. 34 meV, which is again similar to separation between the subsequent sidebands.
The ZPL is likely split as well: in the emission spectra the subcomponent is observed at 578.8 nm line, and in the excitation spectra at 572.9 nm. The observed splitting of the zero-phonon and of vibronic lines might be explained by two possible spin states of the center, i.e., by doublet ground state. In other words, this center is paramagnetic and probably corresponds to one of the centers observed in the EPR spectrum of the studied diamond, which is discussed in the next section.
The second broad PL band with a maximum at 520 nm is the vibronic band of the system with a very weak ZPL at 455 nm (Collins and Mohammed 1982). The energy of the electron-phonons coupling determined from the PL spectrum is 37 meV, and from the PLE spectrum is 38 meV (these values are consistent within the accuracy of the measurements). Previously, the values of 32 meV for the ground and 38.5 meV for the excited states were reported (Pereira and Jorge 1987).
In photoluminescence spectra of all similar diamonds studied before (Collins and Mohammed 1982) the vibronic band of the 455 nm system observed at 405 nm excitation, was markedly distorted by another band with maximum in the region of 540–570 nm. We observe a similar distortion of the band shape, i.e., a significant broadening of long-wavelength edge, in the spectra of the studied diamond under 405 nm excitation. Investigation at variable excitations reveals that the underlying band is the well-known S1 system. Figure 5 shows almost undistorted spectra of the 455 nm system obtained under excitation at 420 nm. The vibronic band of the 455 nm system is almost symmetric in fact. It should be noted that the actual maximum of the band is located near 520 nm and accordingly may cause green luminescence. Yellow luminescence observed previously in similar crystals (Collins and Mohammed 1982) upon excitation with ultraviolet (365 nm) lamp, is obviously due to the S1 center (Fig. 5). The luminescence intensity of the S1 center is close to the intensity of the 455 nm center.
The presence of low-energy vibronic progressions in spectra of the 455 and 575.8 nm centers is attributed (Pereira and Jorge 1987) to the presence of nearly localized vibronic mode, exhibiting a weak coupling to the resonant lattice phonons (the phonon energy of the diamond lattice exceeds 60 meV). Although the models of the centers are not known, the presence of nearly localized vibronic modes may indicate involvement of atoms heavier than carbon.
In addition to the lines described above the photoluminescence spectra of orange diamond specimen shows the N3 and 440 nm centers, and a number of single lines at 418.2, 525.1, 560.8, 645.7 and 799.7 nm. The zero-phonon line at 525.1 nm is characteristic of diamond of cubic habit from Siberia (Zudina et al. 2013). The line at 561 nm was observed in synthetic diamonds (Zaitsev 2001). The line at 799.7 nm was found in the olive type Ib diamonds and was presumably ascribed to nickel (Hainschwang et al. 2013). The ZPL at 645.7 was not reported previously to the best of our knowledge.
The N3 center involving three nitrogen atoms and a vacancy is the most common in natural diamond (Zaitsev 2001). The intensity of this center in PL spectrum of the crystal studied is very low, and perhaps this is why its paramagnetic analogue, the P2 center, was not detected in the EPR spectra.
The 440 nm and S1 centers are characteristic for natural diamonds of cubic habit (Zudina et al. 2013). Their existing models were based on the assumption that they represent the optical analogs of the N3 and OK1 paramagnetic centers, respectively (Zaitsev 2001). Although the sample studied actually contains the N3 and OK1 centers, it has been shown recently (Zudina et al. 2013) that the correlation between the optical centers 440 nm and S1 and paramagnetic centers OK1 and N3, respectively, is not obvious.
The PLE spectra of the orange diamond at liquid nitrogen temperature are shown in Fig. 7. Among them the excitation band of the 575.8 nm center with a maximum at about 490 nm is the most intense. Also the excitation band of the 455 nm center with characteristic vibronic structure and a maximum at about 415 nm is clearly observed. The band of the S1 center with a maximum at 370 nm is poorly visible because of the interference of other bands. In the wavelength range of 330–380 nm, where very broad diffuse band in the absorption spectrum were found, the excitation spectra are rather complicated and consist of broad overlapping bands observing under spectra registration at wide range of light wavelengths. Although these bands are observed also under registration at wavelengths corresponding to the maxima of the vibronic bands of the 455 nm, S1 and 575.8 nm centers, they seem to be not associated with transitions to the highest energy levels of these centers (i.e., the transition to the second excited state from which radiative transitions do not occur), because the intensity of these bands is lower than the intensity of excitation bands due to the transition to the first excited state. However, for the N3 center (blue spectrum in Fig. 7) the PLE band at about 330 nm is less intense than the 390 nm band that may be due to the transition to the first excited state. At the same time the bands revealed in the 320–380 nm region of the excitation spectra might be associated with energy transfer from a center with a high-energy levels to centers with low-energy levels. The nature of these bands requires further special study.
The presence of the bands in the 320–380 nm regions of both PLE and absorption spectra suggest that the well-known absorption continuum at wavelengths <550 nm, is of rather complicated origin and can not be obviously related to a C/P1 center as is widely assumed (Collins 2001; Zaitsev 2001). This conclusion seems to give an answer to the question posed by Hainschwang et al. (2013): why some diamonds with undetectable C center can be strongly colored, i.e. can exhibit the absorption continuum.
Moreover the results obtained show that coloration of the orange diamond specimen is caused not only by absorption, but also by emission of light, i.e. by a luminescence exited by visible light. As it was noted before, almost whole visible region of the spectrum is covered by emission of the various photoluminescence centers: the 575.8 nm center causes red emission; the S1, yellow; the 455 nm, green; the N3 and 440 nm, blue. In our sample, temperature dependence of the intensity of the strongest vibronic band of the 575.8 nm center is very weak. Intensities of the S1 and 455 nm centers decrease considerably at room temperature and can not affect the color. The N3 and 440 nm centers are weak even at liquid nitrogen temperature because of low concentration. Therefore, only the red emission band with maximum at 690 nm arising from the 575.8 nm center upon blue excitation (e.g., 490 nm) contributes appreciably to the coloration of the diamond. Because of this red component of the diamond color varies in intensity depending on the spectral composition of the exciting light (Fig. 1).
We thus conclude that the orange coloration of natural diamonds possessing the 490 nm band in absorption spectra is affected by the red luminescence excited by visible light. This result is in a good agreement with suggestion by Hainschwang et al. (2008). But the diamond studied in that work at nitrogen temperature only seems to exhibit superposition of two luminescence systems, with ZPL’s at 575.8 and 578 nm, which cause rather the orangey yellow emission.
The similar mixing of the absorption and emission contributions were observed for some rare natural green diamonds (Fritsch 1998). They exhibit strong green luminescence of the H3 centers, excited by visible light. The blue luminescence of the N3 centers also may affect the color of a diamond (Collins 2001). These diamonds change coloration with variations in illumination source as well. It is necessary to note that very rare coloration of natural diamonds caused by emission of light looks more brighter and attractive than common absorption-related coloration and is called “precious” in gemology.
The color caused by both absorption and emission of light is rather unusual phenomenon among any other natural minerals (Nassau 2001).
EPR-spectroscopy
The EPR spectra of orange diamond exhibited paramagnetic centers P1, N3, OK1, W24, and some new defects. The crystal studied possess very narrow lines in the EPR-spectra, e.g. only 0.2 Gs for the P1. Such narrow lines were not observed previously in the other natural minerals yet. The diamond studied can be considered as the standard of crystalline perfection that provides a good possibility for study of some paramagnetic centers in more details.
Traditionally, the defect consisting of single substitutional nitrogen is termed C-defect in IR spectroscopy and P1 in EPR studies (Figs. 8, 9). With the EPR spectroscopy this center is detected in almost all natural diamond of octahedral and dodecahedral habit at the level of 0.05–0.5 ppm, but in crystals of cubic habit its contents may reach 60 ppm (Mineeva et al. 2013). In the diamond studied concentration of the P1 centers was 2 ppm, which is lower than the value from IR spectrum (15 ppm). The discrepancy may stems from different causes, in particular from difficulties in precise estimation of the corresponding IR absorption due to complicated spectrum shape in the one-phonon region because of presence of uncommon Y and 1240 cm−1 centers.
In the sample studied unusual behavior of the P1 centers was observed. Two populations of the P1 centers differing in line widths may be distinguished. The P1 defects with narrow lines rapidly saturate with increase of the microwave power applied, which is typical for diamonds. In addition one observes the P1 centers with broader lines, which are, importantly, in the opposite phase. This means that they do not absorb microwave radiation, like other paramagnetic centers, but themselves emit the energy transiting to the ground state. With the increase of microwave power applied the “normal” P1 centers gradually transforms to the P1 centers in the opposite phase. A possible cause of the occurrence of the P1 centers in the opposite phase might be their excited state, that may be due to the energy transfer from another centers.
The paramagnetic centers N3 and OK1 (Fig. 8) are common in natural cubic diamonds (Mineeva et al. 2013). Their contents in the specimen are about 10 and 0.7 ppm respectively. The OK1 and N3 centers involve one substitutional nitrogen atom and another impurity atom. For the OK1 center a C–N–C–O–C chain in the <110> direction in the {110} plane is proposed (Newton and Baker 1989), and for the N3 center, a C–N–O–C chain in the same orientation (van Wyk et al. 1992). Some other models (e.g., Ti-related defects) are less plausible (see Mineeva et al. 2013).
The W24 center has been observed in a few natural yellow diamond crystals only under ultraviolet illumination (van Wyk and Loubser 1983). Based on the data available, it was assumed that the center has trigonal symmetry and is presumably formed by two nitrogens with an unpaired electron localized at the bond. It thus was considered a IR A-defect in a charged state. In crystals with the W24 centers the intensity of the P1 centers signal increases upon UV illumination, thus suggesting co-existence of various charge states of the P1 centers in such crystals. To explain decrease of the luminescence decay time of the P2 centers at high concentrations of the A centers energy transfer between the A and P2 centers was inferred; the presence of P2 centers thus influences the detection probability of the W24. In our orange diamond the P2 center was completely absent and the W24 center was observed without additional light illumination, which allowed analysis of angular dependence of the center lines (Fig. 9). The results obtained showed that the principle axis of the W24 center is oriented along [110] rather than along [111], as previously proposed (van Wyk and Loubser 1983). Two other axes are oriented along [101] and [001] directions, so this center possess a tetragonal symmetry. The parameters of the spin-Hamiltonian of the W24 center with S = 1 are as follows: g = 2.0025, Dx = 132.4 (5) G, Dy = 89.4 (5) G, and Dz = 221.8 (5) Gs. Such a large splitting clearly indicates that the nitrogen atoms are not involved in the center. Most likely the responsible impurity is the phosphorus atoms, which are characterized by similar splitting and by the outer electron shell similar to that of nitrogen. The center likely contains two phosphorus atoms, as evidenced by a spin S = 1. Based on the symmetry of the center it can be proposed that the phosphorus atoms in the center are the second neighbors, i.e. they are separated by a carbon atom. The presence of phosphorus in the environment of natural diamond crystallization is indicated by presence of apatite and other P-rich micro-inclusions (Shiryaev et al. 2005; Titkov et al. 2006; Kaminsky et al. 2013). Previously phosphorus structural impurity has not been reported in natural diamonds to the best of our knowledge, although phosphorus paramagnetic centers were revealed in synthetic diamonds (Nadolinny et al. 2011).
Moreover the principal lines of the P1 center in the diamond studied are accompanied by relatively weak satellite lines with the same width. In the EPR spectra two doublets with splitting 0.7 G (2 MHz) and 0.35 G (1 MHz) (Fig. 10) are observed. The intensity of these lines is many times greater than the intensity of the lines of the 13C isotope allowing their assignment to hyperfine structure of an impurity atom in the nearest neighborhood of the P1 centers. The hyperfine structure corresponds to three equidistant characteristic lines from the 14N isotope. The sidelines are clearly observed, whereas the central line overlaps with the intense line of the fine structure of another nitrogens. We recently reported the first observation of these centers in natural type IaA + Ib diamonds of cubic habit and designated them as the M5 and M6 (Mineeva et al. 2013), in accordance with the rules accepted in EPR spectroscopy of diamonds (M stands for Moscow Institute of geology of ore deposits, petrography, mineralogy and geochemistry of RAS; number denoted the order of identification).
Such a small splitting observed due to the exceptional degree of crystalline perfection of the specimen, indicate that the two nitrogens forming these centers are separated by considerable distance in the structure—ca. 8–10 interatomic spacings, taking into account that the splitting in similar M2 and M3 centers, in which two nitrogens are separated by four interatomic spacings, are significantly higher. The existence of such peculiar extended defects might be due to localization of the impurities on linear defects, e.g. on dislocations.
Another unusual feature of these centers is that the hyperfine parameters of the first nitrogen atom on which the unpaired electron is principally localized, do not differ much from the P1 center. This is in contrast to other similar W7, N1, M2, M3 centers formed by two nitrogens, where the hyperfine parameters of the nearest nitrogen vary considerably and depend on the distance to the second nitrogen (Mineeva et al. 2009). The centers M5 and M6 can be considered as the P1 center in disturbed state due to the weak interaction of its unpaired electron with another N+ located at a considerable distance. Hainschwang et al. (2012) have already suggested that the IR-active Y center may be another form of single nitrogen atoms in the diamond structure. The broad line of the Y center consists of several components. We thus propose that the Y-defect in IR might be an IR manifestations of M5 and/or M6 paramagnetic centers. It is possible that such extended defects might be related to a broad diffuse band in the region of 320–380 nm of the visible optical spectrum of the diamond.
It should be emphasized that the observation of defects such as the M5 and M6 with long-range bonding between impurity atoms is of broad interest. This type of paramagnetic centers was not observed in other minerals and their detection was possible due to simplicity of the diamond structure and a very high degree of crystalline perfection leading to extremely narrow EPR lines.
Besides the lines mentioned above, the EPR spectra of the sample exhibits a set of lines in the area 0.320–0.323 T with g-factors characteristic for Ni defects in the diamond structure (Fig. 9). The spectrum contains the triplet with the splitting characteristic for nitrogen atoms. Therefore, the defect contains both atoms-N and Ni. We were not able to analyze full angular dependence of the center due to its low concentration; useful spectra were recorded in two orientations, along the three- and four-fold axes. The parameters of the center for these directions are: g = 2.18 and A = 12 G (30 MHz) for H ∥ L3, and g = 2.19 and A = 14 G (35 MHz) for H ∥ L4. The center is characterized by S = 1/2, indicating incorporation of one Ni+ ion. The new center formed by atoms of Ni+ and N atoms is designated as M7.
Many Ni-related paramagnetic centers, often also including nitrogen, have been observed in synthetic diamonds (Nadolinny et al. 2005). It was assumed that stable Ni-containing centers with a single N atom, similar to that one found in the studied sample, can not exist in the diamond structure and that only under excitation by light with an energy greater than 3.76 eV the photochromic center NE7 with the hyperfine structure of a nitrogen atom with the constants that vary within 17–25 MHz can be observed (Nadolinny et al. 1999).
Some of the Ni-related centers revealed originally in synthetic diamonds, have been also revealed in natural diamonds, in particular, the NE1 and NE2 (Mineeva et al. 1995, 1996; Titkov et al. 1999; Noble et al. 1998). Some Ni-containing M1 (Mineeva et al. 1995; Titkov et al. 1999) and MP1 centers (Noble et al. 1998) are found in natural diamonds only.
According to EPR-classification of natural diamonds (Mineeva et al. 1995), based on presence of three principal paramagnetic centers P1, P2 or N2, the studied orange diamond should be attributed to the group I, i.e. to the group with predominating P1 defects. However, concentration of the N3 defects in the sample is several times higher than that of P1. This makes the investigated crystal unique among more than 500 natural diamonds studied in our group, in which the P1, P2 or N2 paramagnetic centers always dominated, and the other center occurred in a subordinate amount.
The EPR data allow as to make some conclusions about nature of the 455 and 575.8 nm centers observed in absorption and luminescence spectra of natural orange diamonds. These optical centers are not related to oxygen-related paramagnetic defects OK1 and N3, which were observed earlier in diamond crystals without the 455 and 575.8 nm centers (Zudina et al. 2013). Earlier the 575.8 nm center was ascribed to O+ defect in diamond structure on the base of ab initio plane-wave pseudopotential calculation (Gali et al. 2001). But such a hole center must be in paramagnetic state and exhibit characteristic EPR spectrum, which was not detected in the diamond studied. At the same time the 455 and 575.8 nm centers might represent optical manifestations of the phosphorous-related W24 or nickel-related M7 paramagnetic centers observed. But the concentration of the M7 centers is too low. The relation between the 575.8 nm center, which have the most significant impact on the color of a diamond both in light absorption and in emission, and one of the paramagnetic centers is indirectly supported by the splitting of the vibronic bands in its photoluminescence spectrum, which may be associated with the existence of two spin states. To confirm this suggestion it’s necessary, of course, to conduct additional studies using the technique of optical detection of electron paramagnetic resonance.
Conclusions
Natural orange diamond studied contained a unique set of structural defects, some of which involve nitrogen (A, B, C/P1, Y, M5, M6, N3 centers), hydrogen, nickel (M7 centers), oxygen (OK1 and N3 centers), possibly phosphorus (W24) and vacancies. But only few of revealed defects are responsible for the attractive orange color of the diamond. The coloration is due to both absorption (the broad 320–370 nm band and 490 nm band of the 575.8 nm center) and to emission of light (690 nm vibronic band of the 575.8 nm center), i.e. to luminescence. Luminescent coloration of diamond seems to present a new type of coloration of natural minerals.
References
Boyd SR, Kiflawi I, Woods GS (1994) The relationship between infrared absorption and the A defect concentration in diamond. Phil Mag B 69:1149–1153
Boyd SR, Kiflawi I, Woods GS (1995) Infrared absorption by the B nitrogen aggregate in diamond. Phil Mag B 72:351–361
Collins AT (2001) The colour of diamond and how it may be changed. J Gemmol 27:335–339
Collins AT, Mohammed K (1982) Optical studies of vibronic bands in yellow luminescing natural diamonds. J Phys C Sol St Phys 15:147–158
Dobrinets IA, Vins VG, Zaitsev AM (2013) HPHT-treated diamonds. Springer, Berlin
Fritsch E (1998) The nature of color in diamonds. In: Harlow GE (ed) The nature of diamonds. Cambridge University Press, Cambridge
Gali A, Lowther JE, Deak P (2001) Defect state of subtitutional oxygen in diamond. J Phys Condens Matter 13:11607–11613
Hainschwang T, Notari F, Fritsch E, Massi L (2006) Natural, untreated diamonds showing the A, B and C infrared absorption (“ABC diamonds”) and the H2 absorption. Diam Relat Mater 15:1555–1564
Hainschwang T, Notari F, Fritsch E, Massi L, Rondeau B, Breeding CM, Vollstaedt H (2008) HPHT treatment of CO2 containing and CO2-related brown diamonds. Diam Relat Mater 17:340–351
Hainschwang T, Fritsch E, Notari F, Rondeau B (2012) A new defect centre in type Ib diamond inducting one phonon infrared absorption: the Y centre. Diam Relat Mater 21:120–126
Hainschwang T, Fritsch E, Notari F, Rondeau B, Katrusha A (2013) The origin of color in natural C center bearing diamonds. Diam Relat Mater 39:27–40
Hofer SC (1998) Collecting and classifying colored diamonds: an illustrated study of the Aurora collection. Ashland Press, New York
Kaminsky FV, Khachatryan GK (2001) Characteristics of nitrogen and other impurities in diamond, as revealed by infrared absorption data. Can Mineral 39:1733–1745
Kaminsky FV, Wirth R, Schreiber A (2013) Carbonatitic inclusions in deep mantle diamond from Juina, Brazil: new minerals in the carbonate halide association. Can Mineral 51:669–688
Matsyuk SS, Bartoshinsky ZV, Platonov AN, Bekesha SN, Vinnichenko TG (1990) The optical spectra and the coloration of diamonds from some deposits of the USSR. Mineral Sborn L’vov Univ 44:3–10
Mineeva RM, Speranskiy AV, Titkov SV, Bershov LV (1995) A new type of paramagnetic centre based on Ni ions in natural diamonds. Dokl Earth Sci 335A:143–147
Mineeva RM, Titkov SV, Speranskii AV, Bershov LV (1996) EPR-classification of natural diamonds. Dokl Earth Sci 346:250–253
Mineeva RM, Titkov SV, Speransky AV (2009) Structural defects in natural plastically deformed diamonds: evidence from EPR spectroscopy. Geol Ore Deposits 51:233–242
Mineeva RM, Zudina NN, Titkov SV, Ryabchikov ID, Speransky AV, Zudin NG (2013) EPR-spectroscopy of diamonds of cubic habit from the placers in the North-East of the Siberian platform: new type of nitrogen centers. Dokl Earth Sci 448:243–247
Nadolinny VA, Yelisseyev AP, Baker JM, Newton ME, Twitchen DJ, Lawson SC, Yuryeva OP, Feigelson BN (1999) A study of 13C hyperfine structure in the EPR of nickel-nitrogen-containing centres in diamond and correlation with their optical properties. J Phys: Condens Matter 11:7357–7376
Nadolinny VA, Baker JM, Yuryeva OP, Newton ME, Twitchen DJ, Palyanov YN (2005) EPR study of the peculiarities of incorporating transition metal ions into the diamond structure. Appl Magn Res 28:365–381
Nadolinny VA, Pal’yanov YN, Kalinin AA, Kupriyanov IN, Veber SL, Newton MJ (2011) Transformation of as-grown phosphorus-related Centers in HPHT treated synthetic diamonds. Appl Magn Res 41:371–382
Nassau K (2001) The physics and chemistry of color: the fifteen causes of color, 2nd edn. Wiley, New York
Newton ME, Baker JM (1989) 14 N ENDOR of the OK1 centre in natural type Ib diamond. J Phys Condens Matter 1:10549–10561
Noble CJ, Pawlik T, Spaeth JM (1998) Electron paramagnetic resonance investigations of nickel defects in natural diamonds. J Phys Condens Matter 10:11781–11793
Orlov YuL (1977) The mineralogy of the diamonds. Wiley Intersciece, New York
Pereira E, Jorge MIB (1987) Vibronic coupling to nearly localized modes in diamond. Sol St Commun 61:75–78
Pereira ME, Jorge MIB, Thomaz MF (1986) The red luminescence spectrum of brown diamonds: vibronic coupling. J Phys C Sol St Phys 19:1009–1015
Plotnikova SP, Kluev YuA, Parfianovich IA (1980) Longwave photoluminescence of natural diamonds. Mineral Zhurn 2:75–80
Shiryaev AA, Izraeli ES, Hauri EH, Zakharchenko OD, Navon O (2005) Chemical, optical and isotopic investigation of fibrous diamonds from Brazil. Russ Geol Geophys 46:1185–1201
Titkov SV, Bershov LV, Scandale E, Saparin GV, Chukichev MV, Speranskiy AV (1999) Nickel structural impurities in natural diamonds. In: Gurney JJ, Gurney JL, Pascoe MD, Richardson SH (eds) Proceedings of the VIIth international kimberlite conference, vol 2. Cape Town, Red Roof Desing, pp 319–324
Titkov SV, Gorshkov AI, Solodova YP, Ryabchikov ID, Magazina LO, Sivtsov AV, Sedova EA, Gasanov MD, Samosorov GG (2006) Mineral microinclusions in diamonds of cubic habit from the Yakutian deposits based on analytical electron microscopy data. Dokl Earth Sci 406:1106–1108
Titkov SV, Shigley JE, Breeding CM, Mineeva RM, Zudin NG, Sergeev AM (2008) Natural-color purple diamonds from Siberia. Gems Gemol 44:56–64
van Wyk JA, Loubser JHN (1983) Electron spin resonance of a di-nitrogen centre in Cape yellow type Ia diamonds. J Phys C Sol St Phys 16:1501–1506
van Wyk JA, Loubser JHN, Newton ME, Baker JM (1992) ENDOR and high-temperature EPR of the N3 centre in natural type Ib diamonds. J Phys Condens Matter 4:2651–2662
Wang W, Moses TM (2003) Brown-yellow diamonds with an “amber centre” and pink lamellae. Gems Gemol 39:144–145
Woods GS, Collins AT (1983) Infrared absorption hydrogen complexes in type I diamonds. J Phys Chem Solids 44:471–475
Zaitsev AM (2001) Optical properties of diamond: a data handbook. Springer, Berlin
Zudina NN, Titkov SV, Sergeev AM, Zudin NG (2013) The features of the photoluminescence centers in cubic diamonds of various color from the placers in the North-East of the Siberian platform. Zap Russ Mineral Soc 142:57–72
Acknowledgments
We thank T. Hainschwang and F. V. Kaminsky for constructive reviews, which helped to improve the manuscript. This work was supported by the Earth Science Section of the Russian Academy of Sciences (program no. 2).
Author information
Authors and Affiliations
Corresponding author
Additional information
A. M. Sergeev: Deceased
Rights and permissions
About this article
Cite this article
Titkov, S.V., Mineeva, R.M., Zudina, N.N. et al. The luminescent nature of orange coloration in natural diamonds: optical and EPR study. Phys Chem Minerals 42, 131–141 (2015). https://doi.org/10.1007/s00269-014-0705-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-014-0705-x