Abstract
A multi-anvil device was used to synthesize 24 mg of pure γ-Fe2SiO4 crystals at 8.5 GPa and 1,273 K. The low-temperature heat capacity (C p) of γ-Fe2SiO4 was measured between 5 and 303 K using the heat capacity option of a physical properties measurement system. The measured heat capacity data show a broad λ-transition at 11.8 K. The difference in the C p between fayalite and γ-Fe2SiO4 is reduced as the temperature increases in the range of 50–300 K. The gap in C p data between 300 and 350 K of γ-Fe2SiO4 is an impediment to calculation of a precise C p equation above 298 K that can be used for phase equilibrium calculations at high temperatures and high pressures. The C p and entropy of γ-Fe2SiO4 at standard temperature and pressure (S°298) are 131.1 ± 0.6 and 140.2 ± 0.4 J mol−1 K−1, respectively. The Gibbs free energy at standard pressure and temperature (ΔG° f,298) is calculated to be −1,369.3 ± 2.7 J mol−1 based on the new entropy data. The phase boundary for the fayalite–γ-Fe2SiO4 transition at 298 K based on current thermodynamic data is located at 2.4 ± 0.6 GPa with a slope of 25.4 bars/K, consistent with extrapolated results of previous experimental studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Calorimetric measurements of the heat capacity (C p) of many high-pressure phases are incomplete. The entropy value is critical to thermodynamic calculations of the phase equilibrium relations, and the most precise way to obtain the entropy value is by measuring the heat capacity as a function of temperature. However, high-pressure phases must be synthesized experimentally in a multi-anvil device, and the minimum amount of sample needed for low-temperature heat capacity measurement by conventional low-temperature adiabatic calorimetry (low-TAC, e.g., Robie and Hemingway 1972), which is 10–30 g in general, would take hundreds of experimental runs just to make enough sample, hence prohibiting the heat capacity measurement in practice. Recently, the heat capacity option of the Physical Properties Measurement System (PPMS, produced by Quantum Design®), based on heat-pulse calorimetry, has been shown to give precise low-temperature heat capacity measurements of milligram-sized samples (e.g., Dachs and Bertoldi 2005; Yong et al. 2006).
Fayalite (Fe2SiO4) is one end-member of olivine, the most abundant mineral in the earth’s upper mantle. Numerous experimental studies on Fe2SiO4 have shown that fayalite transforms into a phase with a spinel structure (γ-Fe2SiO4) at pressures higher than 4–5 GPa at 700–1,300 K (e.g., Akimoto et al. 1965, 1967; Inoue 1975; Sung and Burns 1976; Furnish and Bassett 1983; Yagi et al. 1987). The natural occurrence of γ-Fe2SiO4 with Fe/(Fe + Mg) ratios ranging from 0.62 to 0.99 has also been reported in a shocked chondrite meteorite (Xie et al. 2002; Xie and Sharp 2004). The transition in (Mg,Fe)2SiO4 is regarded to play an important role in the formation of seismic discontinuities. Therefore, direct measurement of the thermodynamic properties of γ-Fe2SiO4 and fayalite is of great importance in terms of understanding geophysical applications at mantle conditions. Although the thermodynamic properties of fayalite have been well characterized, those of γ-Fe2SiO4 are still in need of characterization. The enthalpy and high-temperature heat capacity of γ-Fe2SiO4 were measured by Akaogi et al. (1989) and Watanabe (1982), respectively. However, for the aforementioned reason, the low-temperature heat capacity and thus entropy value of γ-Fe2SiO4 was not determined calorimetrically. In this study, the heat capacity data of γ-Fe2SiO4 were measured between 5 and 303 K using the PPMS machine at Salzburg, and the entropy and Gibbs free energy at standard temperature and pressure (STP) were calculated from the measured heat capacity data.
Experimental procedures
Sample synthesis and characterization
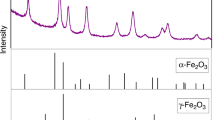
Fayalite was synthesized from a stoichiometric mixture of Fe3O4 and SiO2. After mixing in an agate pestle and mortar, the starting material was reacted at 1,200°C for 48 h in a CO–CO2 gas stream, adjusted to buffer oxygen fugacity at a value of two log units below fayalite–magnetite–quartz equilibrium. A 3-mm diameter Au capsule was loaded with more than 30 mg of fayalite powder and sealed by welding. The γ-Fe2SiO4 used in this study was synthesized from pure fayalite starting material in a 1,000-ton Walker-type multi-anvil device at the University of Minnesota. The experimental assemblage consisted of tungsten carbide anvils with 12 mm truncations, a cast MgO–Al2O3–SiO2–Cr2O3 octahedron, straight-walled graphite heater and internal MgO spacers. The temperature was controlled using a W3Re97/W25Re75 thermocouple, which was located on top of the Au capsule with a 0.35-mm disk of MgO extrusion for electrical isolation. The sample was pressurized to 8.5 GPa before heating at 1,273 K for 12 h. The experiment was quenched by turning off the power to the heater and then slowly depressurized to ambient pressure. The run product was examined by powder X-ray diffraction and electron microprobe (EMP) analysis, and single phase γ-Fe2SiO4 was confirmed.
Heat capacity measurement
The molar heat capacity at constant pressure (C p) of γ-Fe2SiO4 in the temperature range of 5–303 K was measured at 1 atm using the heat capacity option of the PPMS at Salzburg University in Austria. A powdered sample of γ-Fe2SiO4 (24.0 mg) was sealed into a small Al pan with a lid and placed on a 4 mm × 4 mm wide sapphire platform that has a thermometer and a heater attached to the lower side. The C p data were first collected at 60 different temperatures from 5 to 303 K with a logarithmic spacing, and three measurements were performed at each temperature. More C p data were collected around 12 K and from 50 to 303 K with a linear spacing in a second run. A brief summary of this technique is given by Yong et al. (2006), and a more detailed description including discussions of the precision and accuracy of the C p measurement by PPMS is in Lashley et al. (2003) and Dachs and Bertoldi (2005).
Results and discussion
The measured molar heat capacity (C p) data of γ-Fe2SiO4 are listed in Table 1 and plotted in Fig. 1. A C p polynomial of the general form C p = k 0 + k 1 T −0.5 + k 2 T −2 + k 3 T −3 + k 4 T + k 5 T 2 + k 6 T 3 was used to fit the measured molar C p data, which were split into several temperature regions due to the λ-anomaly. The C p data below 5 K were estimated from a plot of C p/T versus T 2 and a linear extrapolation to 0 K. The entropy of γ-Fe2SiO4 at STP is calculated from analytical and stepwise integration of the smoothed C p data and was found to be identical within error when calculated by numerical integration. The resulting entropy value is 140.2 ± 0.4 J mol−1 K−1 (error is two standard deviations). The uncertainty in the entropy is estimated as described by Dachs and Geiger (2006).
In comparison with the C p data of fayalite (Fig. 2, triangles), which has a very sharp λ-transition at 64.9 K as well as a small rounded hump near 16 K (Robie et al. 1982), the C p data of γ-Fe2SiO4 (Fig. 2, circles) exhibit a broad maximum (λ-transition) at 11.8 K and a weak shoulder around 7 K, possibly related to a Schottky-anomaly (e.g., Gopal 1966) arising from the Fe2+ 3d orbitals. The broad λ-transition is likely due to a paramagnetic–antiferromagnetic transition just as the 65 K transition in fayalite (Santoro et al. 1966; Ehrenberg and Fuess 1993). The C p of γ-Fe2SiO4 measured by PPMS is generally lower than that of fayalite (Robie et al. 1982) in the temperature range of 50–300 K (Fig. 2). However, the difference of C p between the two polymorphs decreases with increasing temperature. As a result, the C p data of γ-Fe2SiO4 and fayalite differ less than 1 J mol−1 K−1 at 300 K. If this trend continues to high temperatures, the entropy difference (ΔS) and enthalpy difference (ΔH) for the fayalite–γ-Fe2SiO4 transition will approach constant values, which means the only cause that can change the slope of the transition boundary will be a difference in volume. However, this situation is not supported by the C p data of fayalite and γ-Fe2SiO4 measured between 350 and 700 K by Watanabe (1982), who showed there is a 4–6 J mol−1 K−1 difference between the C p values of these two phases in the range of 350–700 K. Unfortunately, the C p of γ-Fe2SiO4 between 300 and 350 K has not yet been measured and the PPMS routinely measures the C p data only in the range 2–303 K. Therefore a reasonable and precise fitting of C p polynomial above 298 K cannot be undertaken until the discrepancy between the available DSC data and the result of this study is resolved. Until then, an accurate representation of the phase boundary for the fayalite–γ-Fe2SiO4 transition at high pressures and temperatures is cumbersome.
The entropy of γ-Fe2SiO4 at standard pressure and temperature (S°298) calculated from integration of the C p data in this study may be compared with estimates from previous studies. The available estimates of the S°298 of γ-Fe2SiO4 range from 134 to 146 J mol−1 K−1 (e.g., Fei and Saxena 1986; Fei et al. 1991; Fabrichnaya 1998; Jacobs et al. 2001; Jacobs and de Jong 2005). The S°298 value of γ-Fe2SiO4 calculated from the measured C p data in this study, 140.2 ± 0.4 J mol−1 K−1, in the midpoint of the range of estimates, will serve as a strong constraint and substantial improvement for the thermodynamic data base of the Fe2SiO4 system. The enthalpy of the fayalite–γ-Fe2SiO4 transition at standard pressure and temperature (ΔH°tr,298) is given by Akaogi et al. (1989) as 6.6 ± 2.4 kJ mol−1. The enthalpy of formation at 298 K (ΔH° f,298) of fayalite from elements is given by Robie and Hemingway (1995) as −1,478.2 ± 1.3 kJ mol−1. By combining these two studies, the ΔH° f,298 of γ-Fe2SiO4 is calculated to be −1,471.6 ± 2.7 kJ mol−1, which together with our new entropy value, results in the value of Gibbs free energy at standard pressure and temperature (ΔG° f,298), −1,369.3 ± 2.7 kJ mol−1.
The fayalite–γ-Fe2SiO4 transition boundary at 298 K can be calculated using the equation defined as follows:
where \( \Delta H^{ \circ }_{{{\text{tr}},298}} \) and \( \Delta S^{ \circ }_{{{\text{tr}},298}} \) are enthalpy and entropy changes, respectively, for the fayalite–γ-Fe2SiO4 transition at STP, and \( \Delta V(P,298) \) is the volume change at pressure P and at 298 K. The pressure dependence of the molar volume was expressed by the Birch–Murnaghan equation of state:
where K 0 and K′ 0 are the isothermal bulk modulus and its pressure derivative, respectively. The fayalite–γ-Fe2SiO4 transition at 298 K is calculated to be at 2.4 ± 0.6 GPa based on current thermodynamic data given in Table 2. The error of the calculated pressure mainly results from the uncertainties of measured enthalpy change of the transition (Akaogi et al. 1989), with a small contribution from the uncertainties of measured entropies of fayalite and γ-Fe2SiO4 by Robie et al. (1982) and this study, respectively.
The slope of the fayalite–γ-Fe2SiO4 transition at 298 K is also calculated using the Clausius–Clapeyron equation:
where the entropy values at high pressure are corrected with a second order approximation using thermal expansion data at 298 K (Table 2) in the following way:
Substituting P = 2.4 GPa into Eqs. 2–4, the slope of the fayalite–γ-Fe2SiO4 transition at 298 K is calculated to be 25.4 bars/K.
Experimental determinations of the fayalite–γ-Fe2SiO4 transition are plotted and extrapolated to 298 K for comparison with our calculated phase boundary location at 298 K in Fig. 3. Even though most of these experiments were undertaken at high temperatures, between 1,000 and 1,500 K, the extrapolated locations of the transition boundaries by Akimoto et al. (1967) and Yagi et al. (1987) at 298 K agree with our calculation within 0.4 GPa whereas the extrapolated position of the transition by Akimoto et al. (1977) lies at higher pressure. Moreover, the calculated slope of the fayalite–γ-Fe2SiO4 transition, 25.4 bars/K, is in excellent agreement with these experimental studies (Fig. 3), where the slope was given by either 25 bars/K (Akimoto et al. 1977; Yagi et al. 1987) or 26 bars/K (Akomoto et al. 1967). Because of the missing C p data of γ-Fe2SiO4 between 300 and 350 K, a precise calculation of the phase boundary above 298 K cannot be completed at present.
Comparison of the phase transition boundary at 298 K calculated in this study with previous experimental studies. Black square represents our calculated location of phase transition boundary with two standard deviations. The calculated slope at 298 K is also plotted for comparison. A67 Akimoto et al. (1967), A77 Akimoto et al. (1977), Y87 Yagi et al. (1987)
References
Akaogi M, Ito E, Navrotsky A (1989) Olivine-modified spinel–spinel transition in the system Mg2SiO4–Fe2SiO4: calorimetric measurements, thermochemical calculation, and geophysical application. J Geophys Res 94:15671–15685
Akimoto S, Fujisawa H, Katsura T (1965) Olivine–spinel transition in Fe2SiO4 and Ni2SiO4. J Geophys Res 66:1969–1977
Akimoto S, Komada E, Kushiro I (1967) Effect of pressure on the melting of olivine and spinel polymorphs of Fe2SiO4. J Geophys Res 68:679–686
Akimoto S, Matsui Y, Syono Y (1976) High-pressure crystal chemistry of orthosilicates and formation of the mantle transition zone. In: Strens RGJ (eds) The physics and chemistry of minerals and rocks. Wiley, New York, pp 327–363
Akimoto S, Yagi T, Inoue K (1977) High temperature–pressure phase boundaries in silicate systems using in situ X-ray diffraction. In: Manghnani MH, Akimoto S (eds) High-pressure research: application in geophysics. Academic, New York, pp 585–602
Dachs E, Bertoldi C (2005) Precision and accuracy of the heat-pulse calorimetric technique: low temperature heat capacities of milligram-sized synthetic mineral samples. Eur J Mineral 17:251–259
Dachs E, Geiger CA (2006) Heat capacities and entropies of mixing of pyrope–grossular (Mg3Al2Si3O12–Ca3Al2Si3O12) garnet solid solutions: a low-temperature calorimetric and a thermodynamic investigation. Am Mineral 91:8894–9006
Ehrenberg H, Fuess H (1993) Analytical interpretation and simulation of the static magnetic properties of synthetic α-Fe2SiO4. J Phys Condens Matter 5:3663–3672
Fabrichnaya O (1998) The assessment of thermodynamic parameters for solid phases in the Fe–Mg–O and Fe–Mg–Si–O systems. Calphad 22:85–125
Fei Y, Saxena SK (1986) A thermochemical data base for phase equilibria in the system Fe–Mg–Si–O at high pressure and temperature. Phys Chem Miner 13:311–324
Fei Y, Mao H, Mysen BO (1991) Experimental determination of element partitioning and calculation of phase relations in the MgO–FeO–SiO2 system at high pressure and high temperature. J Geophys Res 96:2157–2169
Furnish MD, Bassett WA (1983) Investigation of the mechanism of the olivine–spinel transition in fayalite by synchrotron radiation. J Geophys Res 88:10333–10341
Gopal ESR (1966) Specific heats at low temperatures. Plenum, New York
Inoue K (1975) Development of high temperature and high pressure X-ray diffraction apparatus with energy dispersive technique and its geophysical applications. Ph.D. thesis, Tokyo University, Tokyo
Jacobs MHG, de Jong BHWS (2005) An investigation into thermodynamic consistency of data for the olivine, wadsleyite and ringwoodite form of (Mg,Fe)2SiO4. Geochim Cosmochim Acta 69:4361–4375
Jacobs MHG, de Jong BHWS, Oonk HAJ (2001) The Gibbs energy formulation of α, γ, and liquid Fe2SiO4 using Grover, Getting and Kennedy’s empirical relation between volume and bulk modulus. Geochim Cosmochim Acta 65:4231–4242
Kudoh Y, Takeda H (1986) Single crystal X-ray diffraction study on the bond compressibility of fayalite, Fe2SiO4 and rutile, TiO2 under high pressure. Physica B 139–140:333–336
Lashley JC, Hundley MF, Migliori A, Sarrao JL, Pagliuso PG, Darling TW, Jaime M, Cooley JC, Hults WL, Morales L, Thoma DJ, Smith JL, Boerio-Goates J, Woodfield BF, Stewart GR, Fisher RA, Phillips NE (2003) Critical examination of heat capacity measurements made on a quantum design physical property measurement system. Cryogenics 43:369–378
Marumo F, Isobe M, Akimoto S (1977) Electron-density distributions in crystals of γ-Fe2SiO4 and γ-Co2SiO4. Acta Crystallogr B 33:713–716
Robie RA, Hemingway BS (1972) Calorimeters for heat of solution and low-temperature heat capacity measurement. U.S. Geol Surv Prof Paper 755, 32 pp
Robie RA, Hemingway BS (1995) Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar (105 Pascals) pressure and at higher temperatures. US Geol Surv Bull 2131:461
Robie RA, Finch CB, Hemingway BS (1982) Heat capacity and entropy of fayalite (Fe2SiO4) between 5.1 and 383 K: comparison of calorimetric and equilibrium values of the QFM buffer reaction. Am Mineral 67:463–469
Santoro RP, Newnham RE, Nomura S (1966) Magnetic properties of Mn2SiO4 and Fe2SiO4. J Phys Chem Solids 27:655–666
Sato Y (1977) Equation of state of mantle minerals determined through high-pressure X-ray study. In: Manghnani MH, Akimoto S (eds) High-pressure research: application in geophysics. Academic, New York, pp 307–323
Sung CM, Burns RG (1976) Kinetics of high-pressure phase transformations: implications to the evolution of the olivine–spinel transition in the downgoing lithosphere and its consequences on the dynamics of the mantle. Tectonophysics 31:1–32
Watanabe H (1982) Thermochemical properties of synthetic high-pressure compounds relevant to the earth’s mantle. In: Manghnani MH, Akimoto S (eds) High-pressure research in geophysics. Center for Academic Publications, Japan, pp 441–464
Xie ZD, Sharp TG (2004) High-pressure phases in shock-induced melt veins of the Umbarger L6 chondrite: constraints of shock pressure. Meteorit Planet Sci 39:2043–2054
Xie ZD, Tomioka N, Sharp TG (2002) Natural occurrence of Fe2SiO4–spinel in the shocked Umbarger L6 chondrite. Am Mineral 87:1257–1260
Yagi T, Akaogi M, Shimomura O, Suzuki T, Akimoto S (1987) In situ observation of the olivine–spinel phase transition in Fe2SiO4 using synchrotron radiation. J Geophys Res 92:6207–6213
Yong W, Dachs E, Withers AC, Essene EJ (2006) Heat capacity and phase equilibria of hollandite polymorph of KAlSi3O8. Phys Chem Miner 33:167–177
Acknowledgments
The authors are grateful to C. Henderson for his help in EMP analysis, and R.C. Rouse for his help with XRD measurements. We gratefully acknowledge the constructive reviews of K.-D. Grevel and one anonymous reviewer, which improved the quality of the manuscript. This work was supported by Scott Turner Research Grant by the Department of Geological Sciences, University of Michigan to the senior author, NSF grants EAR 96-28196, 99-11352, 00-87448 and 05-37068 to E.J. Essene, NSF grants EAR 03-10142 and 00-79827 to M. Hirschmann at University of Minnesota, and grant P15880-N11 of the Austrian Science Fund to E. Dachs at the University of Salzburg.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yong, W., Dachs, E., Withers, A.C. et al. Heat capacity of γ-Fe2SiO4 between 5 and 303 K and derived thermodynamic properties. Phys Chem Minerals 34, 121–127 (2007). https://doi.org/10.1007/s00269-006-0133-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-006-0133-7