Abstract
This work describes the mechanochemical synthesis, structural characterization and electrical properties of an interesting group of novel ionic conductors, with general formula Gd2(Hf2−x Ti x )2O7. Different compositions in this system (x = 0, 0.4, 0.8, 1.2, 1.6 and 2) were obtained at room temperature, via a mechanochemical reaction between the corresponding elemental oxides, and characterized by using XRD, Raman spectroscopy and SEM. The XRD structural analysis by the Rietveld method revealed that all the Hf-containing compositions show a disordered fluorite-like structure instead of the expected pyrochlore-like atomic ordering, and the cation size mismatch criteria for pyrochlore stability. Increasing Ti content promotes a phase transformation to the pyrochlore structure with post-milling thermal treatments, which takes place in all samples on annealing at 1200 °C, except for Gd2Hf2O7. These results were confirmed by Raman spectroscopy, which also suggests that the x = 0.4 sample has the highest degree of oxygen disorder in the system and that this disorder decreases with increasing Ti4+ content. Finally, all samples show the pyrochlore structure on firing at 1500 °C. Activation energies E dc for oxygen migration were determined by using impedance spectroscopy and found to be within the ~0.9–1.2 eV range, whereas conductivity σ dc values at 700 °C vary from 1.12 × 10−6 to 2.75 × 10−4 S cm−1, with decreasing conductivity as Ti4+ content increases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lanthanide hafnates Ln2Hf2O7 adopting the pyrochlore and/or fluorite structure belong to a group of complex oxides, which have drawn considerable attention in recent years; in addition to high chemical and structural flexibility, they also show a rich variety of intriguing chemical and physical properties of fundamental and practical importance [1,2,3,4]. In particular, research interest in the ion conducting properties of this family of oxides dates back to the early 1980s, when high oxygen ion mobility at high temperatures was discovered in some Ln2Zr2O7 compounds [5,6,7]. Typical oxygen ion conducting materials such as ZrO2 or CeO2 require doping with aliovalent cations (e.g., Ca2+, Y3+, Ln3+) to create the anion vacancies that support conduction and are therefore extrinsic ionic conductors [8]. By contrast, anion vacancies are inherently present in Ln2B2O7 oxides (Ln = Ln3+; B = tetravalent cation) and conduction does not require the incorporation of dopants; therefore, Ln2B2O7 oxides are intrinsic oxygen ion conductors. Although many different Ln2B2O7 systems (B = Sn, Ti, Zr) have been already synthesized and systematically analyzed [9,10,11,12,13,14], similar HfO2-based complex oxides remain relatively unexplored [15,16,17,18,19,20,21]. This contribution presents a study of the Gd2(Hf2−x Ti x )O7 system; to the best of our knowledge, this is the first time that the mechanochemical synthesis, structural characterization and electrical properties of this solid solution are analyzed. The chemistry of HfO2 and ZrO2 is very similar, and both form analogous complex oxides; however, the physical properties of their derivatives might be substantially different because Hf4+ and Zr4+ have similar ionic radii (R in sixfold coordination = 0.71 vs. 0.72 Å, respectively [22]) but very different electronic density (atomic numbers 72 vs. 40).
Because of the refractory nature of HfO2 and Ln2O3, preparing lanthanide hafnates by the traditional ceramic method requires lengthy firing cycles at very high temperatures (≥1500 °C) [18, 20]. By contrast, all samples analyzed in this work were obtained at room temperature, using a mechanochemical reaction. Mechanochemical methods have become very popular for the synthesis of a wide variety of materials [23, 24]; some of its advantages are the simplicity, the lack of solvents and the possibility of preparing large volumes of the target material using a cost-effective method. Since reactants are processed under non-equilibrium conditions, uncommon and highly defective metastable phases are frequently obtained. Furthermore, additional processing (e.g., post-milling thermal treatments) offers the possibility of isolating fairly stable intermediate states, which are inaccessible for more conventional powder processing techniques.
From the crystalochemical point of view and depending on the R Ln/R B size ratio, Ln2B2O7 oxides will generally adopt either the anion-deficient fluorite structure (R Ln/R B < 1.46) or the pyrochlore structure (R Ln/R B ≥ 1.46) [25]. The well-known ideal fluorite structure can be described as a face-centered cubic array of cations (S.G. = Fm \( \bar{3}\) m (225); Z = 4), with anions placed at all the available tetrahedral interstices. The ideal pyrochlore structure is also isometric (S.G. = Fd \( \bar{3}\) m (227); Z = 8) and might be considered as an ordered derivative of the anion-deficient fluorite structure, i.e., cations and anions are ordered each, in two symmetry-independent sites. Selecting the origin choice 2 of the space group 227, the larger cation (i.e., Ln3+) is generally confined to the 16c Wyckoff position (0,0,0), coordinated by a distorted cuboctahedron of neighboring oxygen sites (two <Ln–O> bonds are significantly shorter than the rest). The smaller cation (B4+) is placed at the 16d site (1/2,1/2,1/2), at the center of a trigonal antiprism, with all six <B–O> bonds of equal length. The anion substructure contains two tetrahedrally coordinated but independent oxygen ions located at the 48f (x,1/8,1/8) and 8a (1/8,1/8,1/8) Wyckoff sites. Therefore, pyrochlore oxides might be better represented as Ln2B2O(1)6O(2) (O(1) = O48f ; O(2) = O8a ). There is also an additional interstitial site in the unit cell, Wyckoff site 8b (3/8,3/8,3/8), easily accessible for anions, which is nominally vacant in ideal pyrochlore structures. As disorder increases (R Ln /R B ≈ 1.46), this site becomes increasingly populated by O48f and O8a ions facilitating oxygen diffusion, and transforming pyrochlore oxides into fast ion conductors. According to calculations [26, 27], the energetically preferred pathway for oxide ion migration will consist of O48f → O48f sequential jumps, along the shortest edges of the BO6 polyhedra. Increasing Ti content in the Gd2(Hf2−x Ti x )O7 system will increase the R Ln /R B size ratio and thus the stability of the pyrochlore structure (i.e., the degree of structural order). The goal of this work is precisely to analyze the effect of the Hf/Ti ratio, on the structural peculiarities and electrical properties of the title solid solution, on samples prepared by using mechanochemical methods.
Materials and methods
Different compositions in the Gd2(Hf2−x Ti x )O7 system (x = 0, 0.4, 0.8, 1.2, 1.6 and 2) were prepared at room temperature, by milling stoichiometric mixtures of the corresponding high purity (≥99%) elemental oxides, baddeleyite-type HfO2, anatase-TiO2 and C-Gd2O3. Milling was carried out in a Retsch PM400 planetary ball mill, using yttria partially stabilized zirconia (5 wt% Y2O3) containers (volume = 125 ml; sample size = 20 g) and grinding media (20 mm ∅; balls-to-powder mass ratio = 10:1; rotation speed = 350 rpm). Phase evolution on milling was followed by using X-ray diffraction (XRD); the reaction time needed to achieve single-phase products was determined by examining at different intervals the XRD pattern of each reaction mixture, i.e., mechanically induced chemical reactions were considered completed when no traces of the starting oxides were evident by this technique. To analyze the relaxation process of mechanically induced defects, as-prepared samples were subjected to post-milling thermal treatments at 1200 and 1500 °C and analyzed as described below.
The structural and microstructural features of the title samples were obtained from precise diffraction data collected by using a Bruker D8 high-resolution X-ray powder diffractometer equipped with a Ge(111) primary monochromator (CuKα1 radiation, λ = 1.5406 Å), and a LynxEye® rapid detector. The angular range, step size and counting times were adjusted to obtain good resolution (the step size should be at least 1/10th of the FWHMs) and statistics. The instrumental contribution to peak broadening was evaluated by using NIST LaB6 standard reference material (SRM 660a; μ = 1138 cm−1, linear absorption coefficient for CuKα1 radiation). The structural refinements were performed by the Rietveld method, using the FullProf program [28]; since no secondary phases were detected by XRD, the chemical composition was included as constraint during the refinements. Therefore, the only free parameters for fluorite-like materials are the unit cell size and the isotropic thermal factors (Debye–Waller), whereas the relative distribution of metal ions for pyrochlore-like phases was allowed to change between the two available sites while maintaining the nominal composition fixed. If needed crystallochemical criteria were used when assigning site occupancy preferences: smaller cations would preferentially occupy the sixfold oxygen-coordinated 16d site; correspondingly, larger ions would tend to sit at the eightfold coordinated 16c site. The samples microstructure was analyzed by using a phenomenological approach described in detail elsewhere [29,30,31,32]. As standard X-ray diffraction techniques are not adequate to study structural features related to light atoms, the oxygen array in our structural refinements is assumed to be ordered, as in the ideal pyrochlore structure, whereas 1/8th of the oxygen positions in fluorite-like phases were considered vacant at random.
The title samples were also characterized by Raman spectroscopy using a Horiba Scientific LabRAM HR Evolution NIR spectrometer, equipped with an Olympus BX41 confocal microscope, a solid-state blue excitation laser (473 nm) and a liquid N2 cooled CCD detector. Raman spectroscopy is especially sensitive to anion disorder in Ln2B2O7 pyrochlore-type oxides, since the spectra are entirely produced by the vibrations of the anion substructure, whereas the Ln and B cations reside in inversion centers (16c and 16d sites symmetry is D 3d ) and generate non-active Raman phonon modes. Powders were also examined by scanning electron microscopy (SEM) using a Philips XL30 ESEM microscope equipped with an EDAX Inc. energy-dispersive X-ray detector for microanalysis.
For the electrical characterization, as-prepared powders were uniaxially pressed (7 Tons cm−2) into pellets (10 mm diameter and ~1 mm thickness) and sintered at 1500 °C for 12 h (heating/cooling rate = 5 °C min−1). The density of the sintered pellets was determined by the Archimedes’ principle, using an Ohaus Discovery DV314C analytical balance (±0.1 mg), equipped with a density determination accessory (P/N 77402-00), and water as immersion medium. AC impedance measurements were carried out in air, as a function of frequency (100 Hz to 1 MHz) and temperature, using a Solartron 1260 frequency response analyzer. Electrodes for impedance spectroscopy were made by coating opposite faces of the pellets, with conductive Pt paste and firing in air at 800 °C to eliminate organic components and harden the Pt residue.
Results and discussion
Mechanochemical synthesis
Figure 1 shows the XRD patterns of a reaction mixture, (1.6 HfO2 + 0.4 TiO2 + Gd2O3), selected as representative of the series: as weighed (1a), and after different milling times (1b). To ease the discussion, the Miller indexes of the most intense reflections, characteristic of each starting chemical, have been labeled in Fig. 1a: baddeleyite-type m-HfO2 (S.G. = P21 /c (14); JCPDS-ICDD PDF34-0104), anatase-TiO2 (S.G. = I41/amd (141); JCPDS-ICDD PDF21-1272) and C-Gd2O3 (S.G. = Ia \( \bar{3} \) (206); JCPDS-ICDD PDF12-0797). Furthermore, the Bragg peaks of the three starting oxides are also shown at the bottom of the same figure. As observed in this figure, even milling for only 1 h produces important changes in the XRD pattern of the starting mixture. The usual effects of decreasing intensity and broadening of all diffraction peaks on milling because of a decreasing particle size, and increasing concentration of structural defects, are more pronounced for anatase-TiO2, and C-Gd2O3, than for m-HfO2, in agreement with the latter higher Moh’s hardness. The characteristic reflections of the first two have disappeared almost completely, after milling for 1 h: the m-HfO2 (\( \bar{1}11 \)) and (111) lines at 28.36° and 31.69° (2θ), respectively, become the most intense of the whole pattern. In addition to these m-HfO2 lines, the XRD pattern obtained after 6 h shows new reflections emerging at for example, ~30 and ~60° (2θ), suggesting that a mechanochemical reaction is taking place. The intensity of these new reflections increases with milling time, and is the only ones observed after 20 h; at this point, the XRD pattern obtained resembles that characteristic of a fluorite-like material. Further milling (10 h) does not produce noticeable changes in the XRD pattern. Figure 1b also shows the Bragg peaks of m-HfO2, and pyrochlore-type Gd2Ti2O7 (JCPDS-ICDD PDF23-0259) and Gd2Zr2O7 (JCPDS-ICDD PDF80-0469); the latter was selected because it is isostructural with Gd2Hf2O7, and there is not a high-quality entry in the JCPDS-ICDD databank for pyrochlore-type Gd2Hf2O7. The absence of additional reflections suggests that mechanical milling is a feasible route to obtain complex oxides in this system, alternative to the traditional solid-state reaction at high-temperature method. Therefore, the same procedure was applied to prepare the remaining samples analyzed in this work. To study the relaxation of mechanochemically induced defects with additional processing, different portions of the as-prepared powders were fired at high temperature and analyzed as described in the previous section.
Structural characterization by XRD and Raman spectroscopy
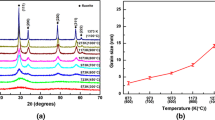
Figure 2 shows the graphical result of fitting the X-ray diffraction patterns of three different Gd2(Hf2−x Ti x )O7 samples: (a) x = 0.4 milled for 30 h, (b) x = 0.8, milled and fired 12 h at 1200 °C and (c) x = 1.2, milled and annealed 12 h at 1500 °C. Table 1 shows the RA/RB size ratio for the title series, whereas the results of the structural refinement plus our samples microstructural features obtained from the Langford plots [29,30,31,32] are collected in Tables 2, 3, 4.
The integral breadth (β) of diffraction lines in samples presenting complex microstructures as the title ones is related to “size” and “microstrain” effects. According to Langford [29, 30] and Halder and Wagner [32], both broadening effects can be separated by using the following equation [1]:
where ε gives the mean apparent domain size and η is a measure of the strain, related to the root mean square strain (e rms) by e rms ~η/5. The graphical representation of equation [1] [(β/d *)2 vs. β/(d *2)] is the so-called Langford plot; the intercept gives the mean value of η, whereas the slope gives ε. Figure 3 shows such plots for the Gd2Hf0.4Ti1.6O7 sample corresponding, respectively, to just-milled powders (3a), and to powders milled and fired 12 h at 1200 °C (3b) and 1500 °C (3c). Worth mentioning is that poor-quality diffraction data obtained for our just-milled Gd2Hf2O7 powders did not allow us to develop a valid and reliable structural model for this particular sample. As a superstructure of an anion-deficient fluorite-like atomic arrangement, the XRD pattern of a pyrochlore-type oxide contains a set of strong peaks characteristic of the fluorite-type substructure, plus an additional set of superstructure reflections [e.g., Miller indexes (111), (311) or (331) lines at ~15°, ~28° and ~38° (2θ)] [33, 34]. The intensity of the latter depends on factors such as the degree of ordering, chemical composition (i.e., the difference in the average scattering factors of the constituent elements) and the distribution of oxygen vacancies. As shown in Table 1, both end members of the Gd2H2−x Ti x O7 system, Gd2Hf2O7 and Gd2Ti2O7, are isostructural and crystallize with the pyrochlore structure; therefore, according to the size ratio criteria for this structure’ stability, the room temperature-stable form of any intermediate composition in the solid solution should be also a pyrochlore-like phase (see Table 1). However, the superstructure peaks are absent in the XRD patterns of our just-milled samples (see for instance Fig. 2a), except for Gd2Ti2O7 [35], i.e., from the XRD point of view, long-range pyrochlore-like atomic ordering is absent in these materials when obtained by using mechanochemical methods. Therefore, they are fluorite-like phases (Table 2) with all metal ions randomly distributed in the cation sublattice [Wyckoff 4a (0,0,0) site]. The lack of metal ordering is most likely a kinetic effect since mechanical milling often produces metastable phases. On the other hand, the only effect of the chemical composition is the expected contraction of the unit cell as the amount of Ti increases (VI R (Ti4+) = 0.605 Å, VI R (Hf4+) = 0.71 Å) [22]. Furthermore, both domain size and internal stress are apparently composition independent (Table 2).
Concerning the effect of post-milling thermal treatments at 1200 °C, the substitution of Hf4+ by Ti4+ enhances, as expected, the stability of the pyrochlore structure. Thus, the Gd2Hf2O7 sample develops into a fluorite-type lattice, whereas increasing Ti4+ content induces the formation of pyrochlore-type (ordered) domains, coexisting with fluorite-like (disordered) ones (Table 3). Both domains grow with increasing Ti content although the ordered ones grow at a faster rate (almost an order of magnitude larger) than the disordered ones. The composition with x = 0.4 seems to be somehow “special” since it was not possible to determine, reliably, the size of the fluorite and pyrochlore domains. At first glance, the XRD pattern of this material appears to correspond to a fluorite-like phase, since the only strong peaks observed are those of the basic unit (sub)cell, common to fluorite and pyrochlore structures. However, the diffraction peaks are rather broad, and the unit cell determined experimentally does not match that of a fluorite structure. A close inspection of the pattern reveals barely some very weak peaks corresponding to the pyrochlore superstructure (Fig. 4); probably, the similarity of scattering powers at both metal sites for this particular composition masks any possible cation ordering effect in the XRD pattern. Therefore, the only peaks clearly observed are those that belong to the fluorite substructure making almost impossible to evaluate any domain size effect on the diffraction peaks. As expected on the basis of crystalochemical considerations, small Ti4+ ions tend to occupy preferentially the BO6 octahedral site displacing the larger Gd3+ and Hf4+ cations to the 16c-site. However, a significant amount of titanium ions are located at the larger 16c-sites, when x > 0.4. In summary, firing these mechanochemically prepared samples at 1200 °C induces cation ordering among the 16c- and 16d-sites, with each ion occupying likely a given position according to its size and crystallochemical properties. However, 1200 °C is not a sufficiently high temperature to reach complete ordering, and some anti-site defects still remain.
Finally, all samples present a pyrochlore-like structure after firing for 12 h at 1500 °C, and the atomic distribution is very close to that of the thermodynamic equilibrium. Indeed, based only on metal ions size, one would expect the large and eight-coordinated 16c site to be fully occupied by Gd3+ ions, whereas the smaller Ti4+ and Hf4+ ions would share the sixfold position (16d). As shown in Table 4, that is the general picture. However, some structural disorder remains even at this temperature. Generally speaking, small Ti ions tend to occupy the 16d-site, whereas the eight-coordinated positions are preferentially occupied by large Gd3+ ions. However, even for high “x” values (high Ti content), a limited but significant amount of Ti4+ ions are placed at the 16c-site; similarly, a non-negligible amount (c.a. 10%) of Gd3+ ions are located in the octahedral 16d-site, with a similar concentration of Hf4+ ions located at the 16c-site. According to a recent structural study on a similar system, Y2Hf2−x Ti x O7, a composition-induced pyrochlore-to-fluorite phase transition takes place when x ≈ 0.5, in agreement with the cation size mismatch criteria [16]: Y2Hf2O7 and Y2Ti2O7 adopt, respectively, the fluorite and pyrochlore structures. However, no such phase transition is observed in the title Gd-containing system on samples fired at high temperature because as mentioned before, both end limits are pyrochlore-type structures.
Concerning these samples microstructure, it should be pointed out that temperature is very effective when releasing the internal structural stress, (Fig. 2c) with minor differences after firing at high temperature, due to material’s composition. By contrast, increasing Ti content seems to favor the growth of the pyrochlore domains, which as shown in Table 4 are considerably larger for the high-temperature fired samples.
As for Raman spectroscopy, selection rules state that fluorites should have only one Raman-active mode of F2g symmetry, with the form of oxygen anions vibrating against the symmetry-fixed cations [36]. A pyrochlore phase should have six Raman-active modes. Five of these modes (A1g + Eg + 3F2g) correspond to O48f ions’ vibrations, and one mode (F2g) to vibrations of the O8a ions [35,36,37].
Raman spectra of the samples annealed at 1200 °C are presented in the left panel of Fig. 5. For x = 0, two strongest bands are observed at 337 and 412 cm−1. By comparison with the Raman data reported for La2Hf2O7 pyrochlore, these bands can be attributed to the F2g + Eg and F2g modes, respectively [38]. Furthermore, the A1g mode of La2Hf2O7 can be located together with one F2g mode at 514 cm−1, and the last expected F2g mode at 538 cm−1 [38]. However, Mandal et al. [39] have also suggested that the last expected F2g mode for Dy2Hf2O7 can be observed near 600 cm−1. Our spectra show a weak band at 543 cm−1 that could be assigned to the A1g mode and a relatively strong band at 602 cm−1 that can be most likely attributed to the F2g mode. The fourth F2g mode cannot be clearly seen, but the spectrum of the x = 0.4 sample shows the presence of a very weak band near 490 cm−1 that might be attributed to this mode.
Figure 5 shows that the observed bands for the x = 0 sample are very broad, much broader than previously reported [38] for pyrochlore-type La2Hf2O7. Furthermore, the A1g mode characteristic of a pyrochlore structure is very weak. This behavior proves that this sample has a fluorite-type structure. Increasing concentration of Ti4+ ions leads to a shift of the 337, 543 and 602 cm−1 bands, to lower frequencies (309, 515 and 577 cm−1 for x = 2), whereas the opposite trend is observed for the 412 and 490 cm−1 bands (for x = 2, the former band shifts to 446 cm−1 and the latter merges with the 515 cm−1 band). Interestingly, the intensity of the A1g mode strongly increases with increasing Ti4+ content. This band is known to be very sensitive to disorder, i.e., increasing structural disorder leads to strong broadening, and decreasing intensity of this band [38, 40]; the observed behavior indicates that doping with Ti4+ leads to the growth of the pyrochlore-like domains, in agreement with the X-ray diffraction data. The spectrum of the x = 2 sample is very similar to the Raman spectrum reported for Gd2Ti2O7 single crystal [37], proving that this sample has a pyrochlore structure with small disorder.
It is worth noting that the x = 0 sample shows the presence of extra, very broad bands near 730 and 830 cm−1. With increasing concentration of Ti4+, the intensity of these bands strongly increases first for x = 0.4, and then decreases when x > 0.4. Such high-frequency bands are related to disorder-activated oxygen vibrations [41]. As discussed by Sanjuán et al. [41], appearance of such bands can be explained assuming partial occupation of the empty 8b site of the pyrochlore structure by oxygen ions and/or by exchanging 16d sites with gadolinium. According to the X-ray diffraction data, the highest concentration of Gd3+ ions at 16d sites is observed for the x = 0.8 sample whereas the x = 0.4 and x = 1.2 samples have comparable amount of Gd3+ at the 16d sites (Table 3). However, the high-frequency bands are the strongest for x = 0.4 and much weaker for x = 1.2. These data show, therefore, that cationic disorder alone cannot explain the observed changes in intensity of the high-frequency bands and that oxygen disorder contributes significantly to this behavior. Therefore, our results suggest that the x = 0.4 sample has the highest degree of oxygen disorder and that this disorder decreases with increasing Ti4+ content.
Annealing of the x = 0 sample at higher temperature, i.e., 1500 °C, leads to moderate narrowing of Raman bands (see Fig. 5, right panel). This behavior indicates that increased annealing temperature promotes more ordered structures. The increased annealing temperature has, however, very weak effect on the Raman spectra measured for the other samples, suggesting weak changes of both cationic and anionic disorders.
Electrical properties
Figure 6 shows two representative examples of our samples microstructure, Gd2Hf0.8Ti1.2O7 (a) and Gd2Hf0.4Ti1.6O7 (b), after pressing and firing at 1500 °C. Table 5 shows the density of such sintered pellets, as determined by the Archimedes method together with that calculated from the XRD data, and the relative density (%). All samples showed a fairly dense microstructure, with increasing Ti content increasing also the grain size. Figure 7 shows some results of the electrical characterization carried out on the title samples: Fig. 7a presents the variation of the real part of the conductivity of the Gd2Hf1.6Ti0.4O7 material, as a function of frequency and temperature (log–log representation), and Fig. 7b shows a Nyquist plot for the same sample (400 °C).
a Variation of the real part of the conductivity of the Gd2Hf1.6Ti0.4O7 material, as a function of frequency and temperature (log–log representation); b Nyquist plot of the same sample at 400 °C; c Arrhenius-type plots for the conductivity in the series; d E dc (solid circles) and σ′ at 700 °C (empty squares), as a function of Ti content
The conductivity behavior with frequency observed in Fig. 7a at low temperatures (<500 °C) can be described by the mathematical expression:
where σ dc is the dc conductivity, A is a temperature-dependent parameter and n is a fractional exponent, typically between 0.6 and 1. This expression is the signature of the so-called “Universal Dielectric Response” (UDR) [42], which is consistent with an (almost) constant conductivity value (σ dc) at low frequencies, and a crossover at high frequencies, to a region of a power law-type dependence with frequency. This behavior has been associated with the existence of cooperative effects in the dynamics of mobile ions [43]. The crossover frequency between both regimes increases with increasing temperature and eventually above 500 °C shifts out of the experimental window. Two semicircles in the impedance plane are clearly observed in Fig. 7b. The one at low frequencies represents the grain boundary effects (capacitance values = 1.16 × 10−8 F cm−1), whereas that at higher frequencies represents the bulk contribution (3.93 × 10−10 F cm−1). Bulk dc conductivities at each temperature can be obtained either from the conductivity value at the frequency plateau in Fig. 7a or from the corresponding Nyquist plots, like the one shown in Fig. 7b. The temperature dependence of the bulk conductivity (σ dc) in the title series was analyzed by using an Arrhenius-type expression of the form (σ dc·T) = σ 0·exp(−E dc/kB·T), where σ 0 and E dc are, respectively, the pre-exponential factor and the activation energy for oxygen ion migration. Figure 7c shows the corresponding graphical representation of the (σ dc·T) dependence with the reciprocal of T, for all the samples analyzed. Dotted lines in this graph represent the experimental data least squares best fit to an Arrhenius-type law confirming that ionic diffusion in the samples is thermally activated; E dc values can be easily calculated from the slope of such linear fits, and are shown in Table 6 together with the conductivity at 700 °C for the whole series. Figure 7d shows a graphical representation of these results as a function of the Ti4+ content. Calculated activation energies for the Hf-rich samples are in general ≈ 1 eV, similar to those reported in similar ion conducting systems [12], whereas E dc for Ti-rich compositions decreases below 0.9 eV. Lower E dc does not translate into higher conductivity and σ dc decreases with increasing Ti4+ content. As structural disorder decreases with increasing Hf substitution, so does the number of mobile charge carriers and thus conductivity. However, the highest conductivity in the system corresponds to the Gd2Hf1.6Ti0.4O7 composition, instead of the one with the highest degree of disorder Gd2Hf2O7. Decreasing number of mobile charge carriers with increasing structural ordering is apparently compensated in this particular composition by a lower activation energy for migration and thus conductivity increases in agreement with previous findings [12, 13].
Conclusions
We have shown that Hf-rich samples in the Gd2(Hf2−x Ti x )2O7 system display fast to moderate ion conducting properties with activation energies on the order of ~1 eV and a peak in conductivity at 700 °C, for x = 0.4; typical σ dc values measured for the oxygen ion conducting material par excellence, YSZ, are ~10−2 S·cm−1 at 800 °C and ~10−1 S·cm−1 at 1000 °C [44]. We have also shown that these otherwise highly refractory materials can be successfully obtained at room temperature via a mechanochemical reaction between the corresponding elemental oxides. According to the X-ray diffraction analysis, as-prepared powders crystallize with a fluorite-like structure, whereas post-milling thermal treatments at high temperature promote a phase transition to the thermodynamically stable, pyrochlore-like structure. As expected, the lattice parameter a in the system decreases with increasing Ti4+ content; the microstructural analysis revealed the presence of two types of domains: disordered fluorite-like domains coexisting in all samples, with ordered pyrochlore-like ones. Both types of domains grow with the level of substitution, but the pyrochlore-type grows at a faster rate (almost an order of magnitude larger) with increasing Ti content.
References
Gardner JS, Gingras MJP, Greedan JE (2010) Magnetic pyrochlore oxides. Rev Mod Phys 82:53–107
Ramirez AP, Hayashi A, Cava RJ, Siddharthan R, Sastry BS (1999) Zero-point entropy in “spin-ice”. Nature 399:333–335
Levi CG (2004) Emerging materials and processes for thermal barrier systems. Curr Opin Solid State Mat Sci 8:77–91
Sickafus KE, Minervini L, Grimes RW, Valdez JA, Ishimaru M, Li F, McClellan KJ, Hartmann T (2000) Radiation tolerance of complex oxides. Science 289:748–751
Van Dijk T, de Vries KJ, Burggraaf AJ (1980) Electrical conductivity of fluorite and pyrochlore LnxZr1-xO2−x/2 (Ln = Gd, Nd) solid solutions. Phys Stat Sol A 58:115–125
Burggraaf AJ, van Dijk T, Verkerk MJ (1981) Structure and conductivity of pyrochlore and fluorite type solid solutions. Solid State Ion 5:519–522
Van Dijk MP, de Vries KJ, Burggraaf AJ (1985) Electrical conductivity and defect chemistry in the system (Tb x Gd1-x )2Zr2O7+y , (0 ≤ x ≤ 1; 0 ≤ y ≤ 0.25). Solid State Ion 16:211–224
Subbarao EC, Maiti HS (1982) Solid electrolytes with oxygen ion conduction. Solid State Ion 11:317–338
Shlyakhtina AV, Fedtke P, Busch A, Kolbanev IV, Barfels T, Wienecke M, Sokolov AE, Ulianov VA, Trounov VA, Shcherbakova LG (2008) Effect of the Ca-doping on the electrical conductivity of oxide ion conductor Yb2Ti2O7. Solid State Ion 179:1004–1008
Xia XL, Ouyang JH, Liu ZG (2010) Electrical properties of gadolinium-europium zirconate ceramics. J Am Ceram Soc 93:1074–1080
Porat O, Heremans C, Tuller HL (1997) Stability and mixed ionic electronic conduction in Gd2(Ti1-x Mo x )2O7 under anodic conditions. Solid State Ion 94:75–83
Díaz-Guillén MR, Moreno KJ, Díaz-Guillén JA, Fuentes AF, Ngai KL, García-Barriocanal J, Santamaría J, León C (2008) Cation size effects in oxygen ion dynamics of highly disordered pyrochlore-type ionic conductors. Phys Rev B 78:104304
Díaz-Guillén JA, Fuentes AF, Díaz-Guillén MR, Almanza JM, Santamaría J, León C (2009) The effect of homovalent A-site substitutions on the ionic conductivity of pyrochlore-type Gd2Zr2O7. J Power Sources 186:349–352
Shlyaktina AV, Shcherbakova LG (2012) New solid electrolytes of the pyrochlore family. Russ J Electrochem 48:1–25
Kumar S, Gupta HC (2012) First principles study of dielectric and vibrational properties of pyrochlore hafnates. Solid State Sci 14:1405–1411
Kong L, Zhang Z, de los Reyes M, Karatchevtseva I, Lumpkin GR, Triani G, Aughterson RD (2015) Soft chemical synthesis and structural characterization of Y2Hf x Ti2−x O7. Ceram Inter 41:5309–5317
Anand VK, Bera AK, Xu J, Herrmannsdörfer T, Ritter C, Lake B (2015) Observation of long-range magnetic ordering in pyrohafnate Nd2Hf2O7: a neutron diffraction study. Phys Rev B 92:184418
Blanchard PER, Liu S, Kennedy BJ, Ling CD (2013) Investigating the local structure of lanthanoid hafnates Ln2Hf2O7 via diffraction and spectroscopy. J Phys Chem C 117:2266–2273
Shlyakhtina AV, Shcherbakova LG (2011) Polymorphism and high-temperature conductivity of Ln2M2O7 (Ln = Sm–Lu; M = Ti, Zr, Hf) pyrochlores. Solid State Ion 192:200–204
Brixner LH (1984) Structural and luminescent properties of the Ln2Hf2O7-type rare earth hafnates. Mat Res Bull 19:143–149
Shlyakhtina AV, Knotko AV, Boguslavskii MV, Stefanovich SY, Kolbanev IV, Larina LL, Shcherbakova LG (2007) Effect of non-stoichiometry and synthesis temperature on the structure and conductivity of Ln(2+x)M(2−x)O(7-x/2) (Ln = Sm–Gd; M = Zr, Hf, x = 0-0.286). Solid State Ion 178:59–66
Shannon R (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst A32:751–767
Zyryanov VV (2008) Mechanochemical synthesis of complex oxides. Russ Chem Rev 77:105–135
Fuentes AF, Takacs L (2013) Preparation of multicomponent oxides by mechanochemical methods. J Mater Sci 48:598–611. doi:10.1007/s10853-012-6909-x
Subramanian MA, Aravamudan G, Subba Rao GV (1985) Oxide pyrochlores—a review. Prog Solid State Chem 15:55–143.
Van Dijk MP, Burggraaf AJ, Cormack AN, Catlow CRA (1985) Defect structures and migration mechanisms in oxide pyrochlores. Solid State Ion 17:159–167
Wilde PJ, Catlow CRA (1998) Defects and diffusion in pyrochlore structured oxides. Solid State Ion 112:173–183
Rodríguez-Carvajal J (1993) Recent advances in magnetic structure determination by neutron powder diffraction, Phys B 19:55–69; See also a report in CPD of IUCr, Newsletter 2001, 26, 12; available at http://www.iucr.org/iucr-top/comm/cpd/Newsletters. The program and manual can be found at http://www-llb.cea.fr/fullweb/powder.htm
Langford JI (1992) The use of the Voigt function in determining microstructural properties from diffraction data by means of pattern decomposition. In: Prince E, Stalick JK (eds) Proceedings of the international conference “Accuracy in Powder Diffraction II”. NIST Special Publication 846 Gaithersburg, MD, USA, pp 110–126
Langford JI (1999) Use of pattern decomposition or simulation to study microstructure: theoretical considerations. In: Snyder RL, Fiala J, Bunge HJ (eds) Defect and microstructure analysis by diffraction, IUCr Monographs on Crystallography 10. Oxford University Press, Oxford, pp 59–81
Louër D (1999) Use of pattern decomposition to study microstructure: practical aspects and applications. In: Snyder RL, Fiala J, Bunge HJ (eds) Defect and microstructure analysis by diffraction, IUCr Monographs on Crystallography 10. Oxford University Press, Oxford, pp 671–697
Halder NC, Wagner CNJ (1966) Analysis of the broadening of powder pattern peaks using variance, integral breadth, and Fourier coefficients on the line profile. Adv X-Ray Anal 9:91–102
Heremans C, Wuensch BJ, Stalick JK, Prince E (1995) Fast-ion conducting Y2(ZryTi1-y)2O7 pyrochlores: neutron Rietveld analysis of disorder induced by Zr substitution. J Solid State Chem 117:108–121
Wuensch BJ, Eberman KW, Heremans C, Ku EM, Onnerud P, Yeo EME, Haile SM, Stalick JK, Jorgensen JD (2000) Connection between oxygen-ion conductivity of pyrochlore fuel-cell materials and structural change with composition and temperature. Solid State Ion 129:111–133
Fuentes AF, Boulahya K, Maczka M, Hanuza J, Amador U (2005) Synthesis of disordered pyrochlores, A2Ti2O7 (A = Y, Gd and Dy), by mechanical milling of constituent oxides. Solid State Sci 7:343–353
Moreno KJ, Fuentes AF, Maczka M, Hanuza J, Amador U, Santamaria J, Leon C (2007) Influence of thermally induced oxygen order on mobile ion dynamics in Gd2(Ti0.65Zr0.35)2O7. Phys Rev B 75:184303
Maczka M, Hanuza J, Hermanowicz K, Fuentes AF, Matsuhira K, Hiroi ZJ (2008) Temperature-dependent Raman scattering studies of the geometrically frustrated Dy2Ti2O7, Gd2Ti2O7 and Er2Ti2O7. J Raman Spectrosc 39:537–544
Garg N, Pandey KK, Murli C, Shanavas KV, Mandal BP, Tyagi AK, Sharma SM (2008) Decomposition of lanthanum hafnate at high pressures. Phys Rev B 77:214105
Mandal BP, Garg N, Sharma SM, Tyagi AK (2006) Preparation, XRD and Raman spectroscopic studies on new compounds RE2Hf2O7 (RE = Dy, Ho, Er, Tm, Lu, Y): pyrochlores or defect fluorite? J Solid State Chem 179:1990–1994
Glerup M, Nielsen OF, Poulsen FW (2001) The structural transformation from the pyrochlore structure, A2B2O7, to the fluorite structure, AO2, studied by Raman spectroscopy and defect chemistry modeling. J Solid State Chem 160:25–32
Sanjuán ML, Guglieri C, Diaz-Moreno S, Aquilanti G, Fuentes AF, Olivi L, Chaboy J (2011) Raman and x-ray absorption spectroscopy of the phase evolution induced by mechanical milling and thermal treatments in R2Ti2O7 pyrochlores. Phys Rev B 84:104207
Jonscher AK (1984) Dielectric relaxation in solids. Chelsea Dielectric Press, London
Moreno KJ, Fuentes AF, Amador U, Santamaría J, León C (2007) Influence of structural disorder on the dynamics of mobile oxygen ions in Dy2(Ti1-yZry)2O7. J Non-Cryst Solids 353:3947–3955
Aguadero A, Fawcett L, Taub S, Woolley R, Wu K-T, Xu N, Kilner JA, Skinner SJ (2012) Materials development for intermediate-temperature solid oxide electrochemical devices. J Mater Sci 47:3925–3938. doi:10.1007/s10853-011-6213-1
Acknowledgements
This work was financially supported by CONACYT (Grants CB2013-01-221701 and CB2011-01-166995). The authors acknowledge Prof. Maik Lang for providing the opportunity to carry out the Raman spectroscopy measurements at the University of Tennessee (Knoxville, TN), and Igor M. Gussev for his help when collecting the spectra.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Cepeda-Sánchez, N.M., Díaz-Guillén, J.A., Maczka, M. et al. Mechanochemical synthesis, crystal structure and ion conduction in the Gd2Hf2−x Ti x O7 system. J Mater Sci 52, 11933–11946 (2017). https://doi.org/10.1007/s10853-017-1037-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1037-2