Abstract
Background
To evaluate the outcome of duodenopancreatic reoperations in patients with multiple endocrine neoplasia type 1 (MEN1).
Methods
MEN1 patients who underwent reoperations for duodenopancreatic neuroendocrine neoplasms (dpNENs) were retrieved from a prospective database and retrospectively analyzed.
Results
Twelve of 101 MEN1 patients underwent up to three reoperations, resulting in a total of 18 reoperations for dpNEN recurrence. Patients initially underwent either formal pancreatic resections (n = 7), enucleations (n = 3), or duodenotomy with lymphadenectomy for either NF-pNEN (seven patients), Zollinger–Ellison syndrome (ZES, three patients), organic hyperinsulinism (one patient) or VIPoma (one patient). Six patients had malignant dpNENs with lymph node (n = 5) and/or liver metastases (n = 2). The indication of reoperations was NF-pNEN (five patients), ZES (five patients), organic hyperinsulinism (one patient), and recurrent VIPoma (one patient). Median time to first reoperation was 67.5 (range 6–251) months. Five patients required a second duodenopancreatic reoperation for 60–384 months after initial surgery, and one patient underwent a third reoperation after 249 months. The rate of complications (Clavien–Dindo ≥3) was 28%. Four patients required completion pancreatectomy. Six patients developed pancreoprivic diabetes. After a median follow-up of 18 (6–34) years after initial surgery, ten of 12 patients are alive, one died of metastatic pancreatic VIPoma, and one died of metastatic thymic NEN.
Conclusion
Reoperations are frequently necessary for dpNEN in MEN1 patients, but are not associated with an increased perioperative morbidity in specialized centers. Organ-sparing resections should be preferred as initial duodenopancreatic procedures to maintain pancreatic function and avoid completion pancreatectomy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Duodenopancreatic neuroendocrine neoplasms (dpNENs) represent the most common syndrome-associated cause of death in patients with multiple endocrine neoplasia type 1 (MEN1) [1]. They develop in 60–80% of MEN1 patients and are therefore the second most common manifestation of the syndrome. They also represent one of the most common reasons for morbidity, either by causing a hormonal syndrome when functioning, or in case of metastatic disease, or by postoperative endocrine or exocrine dysfunction [1,2,3,4].
Surgery is indicated for either functioning dpNEN or non-functioning (NF)-pNEN exceeding a critical size of 10–20 mm, as their risk of metastatic spread increases significantly [5,6,7,8,9]. It must be kept in mind that the tumor size differs between different imaging techniques and histopathology [10].
Since the majority of MEN1 patients have multifocal duodenopancreatic disease and NF-pNENs <20 mm carry a low oncological risk [6, 11], nowadays most experts advocate an organ-sparing procedure for the removal of MEN1-associated dpNEN. However, there is an ongoing debate on the indication and extent of surgery [12, 13], and some institutions still promote prophylactic subtotal or total pancreatectomy [14, 15].
Although there are several retrospective studies reporting the outcome for initial surgery of MEN1-associated dpNEN [3,4,5, 16, 17], there is a lack of information regarding the indication and outcome of duodenopancreatic reoperations in MEN1. In 2004, Hausman et al. [18] reported 39 MEN1 patients, 15 of whom underwent reoperations. There was no information on perioperative complications in this series, and as operations in this cohort dated back as far as 1967, the indication of surgery was either a hormonal syndrome or advanced, often metastatic NF-pNENs. Another publication from the same group reported the need for completion pancreatectomy for recurrent Zollinger–Ellison syndrome in eight of 49 patients who underwent an extensive initial resection, including distal pancreatic resection, enucleation from the pancreatic head, and duodenotomy (“Thompson procedure”) [19].
As clinical and pathological data have emerged over the past years and diagnostic techniques are becoming more precise, the understanding of the MEN1 syndrome and its treatment has improved. Clinical practice guidelines by experts [8] and ENETS consensus guidelines [20] are provided for the management of duodenopancreatic disease in MEN1 patients, but recommendations for reoperations are scarce due to the lack of data.
Therefore, the aim of the present study was to evaluate the indications, frequency, complications, and morbidity of reoperations for MEN1-associated dpNENs.
Methods
Data of all genetically confirmed MEN1 patients treated at the Department of Surgery of the Philipps University of Marburg have been prospectively collected in a clinical database since 1997. Previous treatments were retrospectively included to the database. The collected data included patients’ history, family history, results of diagnostic imaging, laboratory tests, surgical and medical treatments as well as histopathology. Data acquisition and evaluation were performed after obtaining the patients’ written informed consent.
Routine screening and follow-up were performed yearly following a standardized protocol, which was adapted to current scientific advances and/or guidelines over the years [21]. Annual screening of dpNEN always included at least the measurement of proinsulin, insulin, gastrin, vasoactive intestinal peptide (VIP), pancreatic polypeptide, and chromogranin A. Functional tests, such as fasting test or secretin stimulation test, were performed, when there was the suspicion of either organic hyperinsulinism or Zollinger–Ellison syndrome (ZES). Annual imaging of the upper abdomen comprised endoscopic ultrasonography (EUS) and in most cases magnetic resonance imaging (MRI) or rarely computed tomography (CT). In cases with suspicion of malignancy, somatostatin receptor scintigraphy was performed until December 2013. Between 2014 and 2016, all patients had Ga68-DOTATOC-PET/CT during a prospective study [22], and since 2017, Ga68-DOTATOC-PET/CT has been performed only in patients with suspicion of malignancy or metastases.
All MEN1 patients with ZES underwent laparotomy after diffuse liver metastases were excluded by preoperative imaging. Bidigital palpation of the pancreas and intraoperative ultrasonography were performed in all patients. Until 1997, the routine initial procedure for ZES was duodenotomy with excision of any tumors in the first to fourth portion of the duodenum (further referred to as “duodenotomy”), peripancreatic lymph node dissection, and enucleation of any pNEN in the pancreatic head with distal pancreatectomy to the level of the portal vein. Since 1998, partial pancreaticoduodenectomy (PPD) with lymphadenectomy was routinely performed as initial procedure [23]. A reoperation was only indicated in case of positive imaging results and a localized pattern of disease.
Patients with organic hyperinsulinism were operated, if the diagnosis was proven by a positive fasting test and diffuse metastatic disease was excluded by imaging. MEN1 patients with NF-pNENs were scheduled for pancreatic resection until 2010, if the tumor size exceeded 10 mm in preoperative imaging and diffuse liver metastases were excluded. Since 2011, NF-pNENs were operated according to ENETS guidelines, if the largest tumor diameter reached 20 mm. In addition, we scheduled patients with NF-pNENs sized between 10 and 20 mm for surgery, if more than 20% growth was observed during 12-month follow-up and/or a CHES1 LOI was detected in the particular patient [24]. For both NF-pNEN and insulinoma, the preferred standard procedure until 2011 was a spleen-preserving left pancreatectomy to the level of the portal vein with enucleation of tumors out of the pancreatic head, if present. Since 2012, parenchyma-preserving resections focussing only on tumors >1–2 cm, such as enucleations or distal pancreatic resections, were preferred, if technically feasible. The indication of reoperations was the same as of primary surgery. The specific reoperation performed depended on the pattern of disease recurrence as visualized by imaging studies.
The database was screened for patients who underwent duodenopancreatic reoperations. Demographics, type of tumors, indications, and type of primary and consecutive duodenopancreatic resections, disease recurrence or new manifestations, complications, and long-term outcome were retrospectively analyzed in identified patients.
The grading system proposed by the International Study Group of Pancreatic Fistula (ISGPF) [25] was used to classify postoperative pancreatic fistulas (POPFs). Delayed gastric emptying as well as post-pancreatectomy hemorrhage was defined according to the proposed definitions of the International Study Group of Pancreatic Surgery (ISGPS) [26]. Postoperative complications were defined according to the Clavien–Dindo classification system [27]. Endocrine pancreatic function was documented by the need for oral antidiabetic medication, insulin therapy, and HbA1c. Malignancy was defined by the existence of metastases. Tumors were graded according to the WHO 2010 classification as G1, G2, or G3.
Results
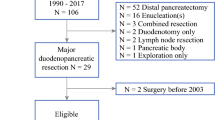
Over a 36-year period, 59 of 101 (58%) genetically confirmed MEN1 patients underwent primary duodenopancreatic resections for dpNEN at our institution. Twelve of these 59 (20.3%) patients underwent up to three duodenopancreatic reoperations, resulting in a total of 18 reoperations after a median follow-up of 18 (6–34) years after initial surgery.
The median age of the 12 patients (five females, seven males) was 35 (range 23–47) years at the time of initial operation. The indication of the primary duodenopancreatic resection was NF-pNEN in seven patients, ZES in three patients, organic hyperinsulinism in one patient, and VIPoma in the remaining patient. The initial duodenopancreatic resection comprised partial pancreaticoduodenectomy (n = 1), distal pancreatectomy to the level of the mesentericoportal axis (n = 6, two laparoscopic, one with synchronous partial adrenalectomy), pancreatic tail resection with (n = 1) or without duodenotomy (n = 1), duodenotomy only (n = 1), or enucleation (n = 2). Four of 12 patients developed postoperative clinically relevant (Clavien–Dindo ≥3) complications, all due to postoperative pancreatic fistula type B. Mortality was nihil. Histopathology detected malignant dpNENs in four of 12 patients (two malignant duodenal ZES, one malignant NF-pNEN, and one malignant VIPoma) based on lymph node (n = 4) and/or liver metastases (n = 1). All the 12 patients had G1 tumors. Two of 12 patients developed insulin-dependent diabetes mellitus after their initial operation, and one patient needed oral antidiabetics. Two of three patients with ZES, the patient with organic hyperinsulinism, and the patient with the malignant VIPoma were biochemically cured at discharge. Characteristics of reoperated patients are given in Table 1.
After a median time of 67.5 (range 6–251) months, a duodenopancreatic reoperation was indicated in these 12 patients either for newly developed NF-pNENs in five patients, for recurrent ZES in three patients, for newly developed ZES in two patients, for organic hyperinsulinism in one patient, or for a local recurrence of VIPoma in one patient, respectively (Table 2). In reoperations, parenchyma-sparing operations were preferred when technically feasible and oncologically justified. Operations included completion pancreatectomy in one patient, distal pancreatic resection in three patients (two with enucleation and one with duodenotomy), duodenotomy with lymphadenectomy in three patients (two combined with pNEN enucleation out of the pancreatic head), partial pancreaticoduodenectomy in one patient, and pNEN enucleation only in four patients, respectively. All reoperations were performed via an open approach. Five of 12 patients had postoperative clinically relevant (Clavien–Dindo ≥3) complications, all caused by pancreatic fistula type B. Mortality was nihil. Histopathology revealed malignancy in five patients (three with lymph node metastases and two with liver metastases). Two of the five patients with ZES and the patients with organic hyperinsulinism were biochemically cured at discharge after the reoperation. The patient with recurrent VIPoma had biochemically persistent disease. Another three patients developed postoperative diabetes mellitus requiring insulin therapy, resulting in five of 12 after the reoperations.
A second reoperation was indicated in five patients 60, 62, 84, 103, and 384 months after their first reoperation for newly developed or grown NF-pNEN >20 mm (one patient), recurrent ZES (two patients), or newly developed ZES (two patients). Partial pancreaticoduodenectomy was performed in three patients, enucleation and lymphadenectomy in one patient, and distal pancreatic resection plus duodenotomy and enucleation from the pancreatic head in another one patient. Partial pancreaticoduodenectomy equaled completion pancreatectomy in two patients. None of these patients had postoperative clinically relevant (Clavien–Dindo ≥3) complications, but three patients newly developed diabetes mellitus requiring insulin therapy.
One female patient (patient no. 6) with a newly developed insulinoma in the pancreatic tail underwent completion pancreatectomy at the age of 58 years as a third reoperation after two duodenotomies with excision of duodenal wall gastrinomas and partial pancreaticoduodenectomy for recurrent ZES. The postoperative course was uneventful, despite the development of pancreoprivic diabetes mellitus. The patient was biochemically cured 90 months after the last operation.
After a median follow-up period of 216 (range 78–408) months [=18 (range 6–34) years] after initial surgery, ten of the 12 reoperated patients are alive, one patient died of metastatic pancreatic VIPoma 20 years after initial surgery, and one patient died of metastatic thymic NEN 13 years after initial surgery for ZES. Nine patients developed pancreoprivic diabetes requiring insulin therapy. Postoperative complications were exclusively pancreatic fistulas, and there was no postoperative mortality. Details of reoperations are summarized in Table 2.
Discussion
A majority of MEN1 patients harbor diffuse islet cell hyperplasia and multiple NEN in the pancreas [7, 28]. Therefore, long-term cure can only be achieved by pancreatectomy. However, this procedure goes along with severe long-term morbidity. Therefore, it is crucial for MEN1 patients to find a compromise between the preservation of pancreatic function and the cure of any hormonal excess caused by dpNENs as well as the prevention of metastatic dpNEN disease. Current practice guidelines recommend the surgical removal of functioning pNEN and non-functioning pNEN with significant growth over 3–6 months or those exceeding 10–20 mm [8]. There has been a recommendation toward less aggressive indication of surgery during the past years [20], and it could be shown in a large multicentric retrospective series that pNENs <20 mm pose a low oncological risk [29].
An exception is made by MEN1–gastrinomas causing Zollinger–Ellison syndrome (ZES), as they—unlike believed for many years—arise from the duodenal wall and are often multiple and lymphatic metastases are frequently apparent at the time of diagnosis [30].
The type of surgery is highly controversial and depends on the type and location of dpNEN. In the past, aggressive surgical treatment and prophylactic pancreatic resections were promoted in order to prevent metastatic tumor growth in many centers with expertise in MEN1 including our institution [4, 14, 18]. However, data proving a prophylactic benefit in regard to long-term survival and quality of life are pending. As earlier data from our institution showed that also with more aggressive surgical strategies, the rate of recurrent pancreaticoduodenal disease was high, did not lead to metastatic disease, but made further pancreatic resections necessary with loss of organ function, we have been following a less aggressive strategy regarding both indications and extent of surgery [4]. Small NF-pNEN and insulinomas in MEN1 patients will be enucleated whenever technically feasible, which is mainly dependent on their localization with regard to the major pancreatic duct. In case of MEN1-ZES with additional pNENs in the pancreatic tail, a partial pancreaticoduodenectomy with enucleation or spleen-preserving pancreatic tail resection will be considered. This strategy also prevents the development of metastatic disease and saves organ function, although it might result in a higher risk of recurrence of pNENs with a need for reoperations. However, the presented results support the current concept of a less aggressive surgical approach and points to continue with it. In order to perform an optimal pancreas-sparing surgical treatment in MEN1-associated pdNEN, detailed knowledge of the disease and a high expertise in open and minimally invasive pancreatic surgery is required.
Reoperations, especially of the pancreas, are considered technically demanding with a high risk of complications. This might be especially true for MEN1 patients, who usually have a very soft pancreas with a small pancreatic duct, which are major risk factors for postoperative severe complications, especially POPF and postoperative pancreatic hemorrhage. Unfortunately, there are almost no data regarding the outcome of duodenopancreatic reoperations in MEN1. The present study is the third focusing on this issue, while the two previous publications came from the same institution [18, 19]. The previous studies reported a high risk of recurrent pdNEN with the need for reoperations (62% of initially operated patients [18]) and a frequent need for completion pancreatectomies, especially in patients with ZES after initial extensive resections [19].
Our present data show that duodenopancreatic reoperations in MEN1 patients do not harbor an increased risk of neither perioperative complications nor morbidity compared with the initial resection. Thus, we would encourage to initially perform organ-sparing resections, whenever oncologically and technically feasible to maintain as much pancreatic function as possible and thus quality of life. As shown in the present series, this philosophy can avoid a completion pancreatectomy even after repeated reoperations for a long time, without impairing patients’ prognosis. Also, reoperations were performed after a median of more than 5 years, and some patients did not require operative treatment for dpNEN recurrence for more than 30 years. Endocrine and exocrine pancreatic malfunctions can thus be delayed for a long time, even if further pancreatic resections become necessary.
Conclusion
Reoperations are frequently necessary for duodenopancreatic NEN in MEN1 patients, but are not associated with an increased perioperative morbidity in specialized centers. Therefore, organ-sparing resections should be preferred as initial duodenopancreatic procedures to maintain pancreatic function and avoid completion pancreatectomy as long as possible.
References
Goudet P, Murat A, Binquet C et al (2010) Risk factors and causes of death in MEN1 disease. A GTE (Groupe d’Etude des Tumeurs Endocrines) cohort study among 758 patients. World J Surg 34:249–255
Gibril F, Venzon DJ, Ojeaburu JV et al (2001) Prospective study of the natural history of gastrinoma in patients with MEN1: definition of an aggressive and a nonaggressive form. J Clin Endocrinol Metab 86:5282–5293
Ito T, Igarashi H, Uehara H et al (2013) Causes of death and prognostic factors in multiple endocrine neoplasia type 1: a prospective study: comparison of 106 MEN1/Zollinger–Ellison syndrome patients with 1613 literature MEN1 patients with or without pancreatic endocrine tumors. Medicine (Baltimore) 92:135–181
Lopez CL, Waldmann J, Fendrich V et al (2011) Long-term results of surgery for pancreatic neuroendocrine neoplasms in patients with MEN1. Langenbecks Arch Surg 396:1187–1196
Triponez F, Dosseh D, Goudet P et al (2006) Epidemiology data on 108 MEN 1 patients from the GTE with isolated nonfunctioning tumors of the pancreas. Ann Surg 243:265–272
Triponez F, Goudet P, Dosseh D et al (2006) Is surgery beneficial for MEN1 patients with small (< or = 2 cm), nonfunctioning pancreaticoduodenal endocrine tumor? An analysis of 65 patients from the GTE. World J Surg 30:654–662 discussion 663-664
Anlauf M, Schlenger R, Perren A et al (2006) Microadenomatosis of the endocrine pancreas in patients with and without the multiple endocrine neoplasia type 1 syndrome. Am J Surg Pathol 30:560–574
Thakker RV, Newey PJ, Walls GV et al (2012) Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J Clin Endocrinol Metab 97:2990–3011
Newey PJ, Jeyabalan J, Walls GV et al (2009) Asymptomatic children with multiple endocrine neoplasia type 1 mutations may harbor nonfunctioning pancreatic neuroendocrine tumors. J Clin Endocrinol Metab 94:3640–3646
Polenta V, Slater EP, Kann PH et al (2018) Preoperative Imaging Overestimates the tumor size in pancreatic neuroendocrine neoplasms associated with multiple endocrine neoplasia type 1. World J Surg 42:1440–1447
Partelli S, Tamburrino D, Lopez C et al (2016) Active surveillance versus surgery of nonfunctioning pancreatic neuroendocrine neoplasms ≤2 cm in MEN1 patients. Neuroendocrinology 103:779–786
Jensen RT, Norton JA (2017) Treatment of pancreatic neuroendocrine tumors in multiple endocrine neoplasia type 1: some clarity but continued controversy. Pancreas 46:589–594
Ito T, Hijioka S, Masui T et al (2017) Advances in the diagnosis and treatment of pancreatic neuroendocrine neoplasms in Japan. J Gastroenterol 52:9–18
Akerstrom G, Hessman O, Skogseid B (2002) Timing and extent of surgery in symptomatic and asymptomatic neuroendocrine tumors of the pancreas in MEN 1. Langenbecks Arch Surg 386:558–569
Adkisson CD, Stauffer JA, Bowers SP et al (2012) What extent of pancreatic resection do patients with MEN-1 require? JOP 13:402–408
Bartsch DK, Albers M, Knoop R et al (2013) Enucleation and limited pancreatic resection provide long-term cure for insulinoma in multiple endocrine neoplasia type 1. Neuroendocrinology 98:290–298
Nell S, Borel Rinkes IHM, Verkooijen HM et al (2018) Early and late complications after surgery for MEN1-related nonfunctioning pancreatic neuroendocrine tumors. Ann Surg 267:352–356
Hausman MS Jr, Thompson NW, Gauger PG et al (2004) The surgical management of MEN-1 pancreatoduodenal neuroendocrine disease. Surgery 136:1205–1211
Gauger PG, Doherty GM, Broome JT et al (2009) Completion pancreatectomy and duodenectomy for recurrent MEN-1 pancreaticoduodenal endocrine neoplasms. Surgery 146:801–806 discussion 807-808
Falconi M, Eriksson B, Kaltsas G et al (2016) ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology 103:153–171
Manoharan J, Albers MB, Bartsch DK (2017) The future: diagnostic and imaging advances in MEN1 therapeutic approaches and management strategies. Endocr Relat Cancer 24:T209–T225
Albers MB, Librizzi D, Lopez CL et al (2017) Limited value of Ga-68-DOTATOC-PET-CT in routine screening of patients with multiple endocrine neoplasia type 1. World J Surg 41:1521–1527
Lopez CL, Falconi M, Waldmann J et al (2013) Partial pancreaticoduodenectomy can provide cure for duodenal gastrinoma associated with multiple endocrine neoplasia type 1. Ann Surg 257:308–314
Bartsch DK, Slater EP, Albers M et al (2014) Higher risk of aggressive pancreatic neuroendocrine tumors in MEN1 patients with MEN1 mutations affecting the CHES1 interacting MENIN domain. J Clin Endocrinol Metab 99:E2387–2391
Bassi C, Dervenis C, Butturini G et al (2005) Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 138:8–13
Panwar R, Pal S (2017) The international study group of pancreatic surgery definition of delayed gastric emptying and the effects of various surgical modifications on the occurrence of delayed gastric emptying after pancreatoduodenectomy. Hepatobiliary Pancreat Dis Int 16:353–363
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Kann PH, Kann B, Fassbender WJ et al (2006) Small neuroendocrine pancreatic tumors in multiple endocrine neoplasia type 1 (MEN1): least significant change of tumor diameter as determined by endoscopic ultrasound (EUS) imaging. Exp Clin Endocrinol Diabetes 114:361–365
Partelli S, Tamburrino D, Lopez C et al (2016) Active surveillance versus surgery of nonfunctioning pancreatic neuroendocrine neoplasms ≤2 cm in MEN1 patients. Neuroendocrinology 103:779–786
Bartsch DK, Albers MB (2015) Controversies in surgery for multiple endocrine neoplasia type 1-related Zollinger–Ellison syndrome. Int J Endocr Oncol 2:71–263
Acknowledgements
We thank all MEN1 patients participating in our controlled screening program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Albers, M.B., Manoharan, J., Bollmann, C. et al. Results of Duodenopancreatic Reoperations in Multiple Endocrine Neoplasia Type 1. World J Surg 43, 552–558 (2019). https://doi.org/10.1007/s00268-018-4809-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-018-4809-1