Abstract
Background
Routine screening is recommended for patients with multiple endocrine neoplasia type 1 (MEN1) to enable early detection and treatment of associated neuroendocrine neoplasms (NEN). Gallium68-DOTATOC-Positron emission tomography combined with computed tomography (Ga-68-DOTATOC-PET-CT) is a very sensitive and specific imaging technique for the detection of sporadic neuroendocrine tumors. The present study evaluated the value of Ga-68-DOTATOC-PET-CT in routine screening of patients with MEN1.
Methods
Between January 2014 and March 2016, all MEN1 patients underwent Ga-68-DOTATOC-PET-CT in addition to conventional imaging (computed tomography of the thorax, magnetic resonance imaging of the abdomen and pituitary, endoscopic ultrasonography). The diagnostic yield of conventional imaging and Ga-68-DOTATOC-PET-CT was prospectively documented and compared, and treatment changes caused by the addition of Ga-68-DOTATOC-PET-CT were recorded.
Results
Conventional imaging detected 145 NENs, mainly pancreaticoduodenal NENs (n = 117, 81%), in 31 of 33 MEN1 patients. Ga-68-DOTATOC-PET-CT detected 55 NENs in 23 of the 33 patients (p = 0.0001). Ninety (62%) NENs detected by conventional imaging were missed by DOTATOC-PET-CT. The majority of missed lesions were pNEN (n = 68; 74%). The sensitivity of Ga-68-DOTATOC-PET-CT for NENs <5, 5–9, 10–19 and ≥20 mm was 0, 29, 81 and 100%, respectively. However, Ga-68-DOTATOC-PET-CT detected more liver and lymph node metastases in patients with known metastatic disease, which did not lead to a change of patients’ management. In one patient (3%), Ga-68-DOTATOC-PET-CT was the only imaging modality that detected a small intestine NEN and led to potentially curative surgery.
Conclusion
Ga-68-DOTATOC-PET-CT cannot be recommended for routine screening of MEN1 patients. It might provide important additional information in patients with suspected or known metastatic disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Multiple endocrine neoplasia type 1 (MEN1) is an autosomal dominant neuroendocrine tumor syndrome caused by germline mutations of the tumor suppressor gene MENIN on chromosome 11q13. The MEN1 syndrome is characterized by the manifestation of gastroenteropancreatic neuroendocrine neoplasms (GEP–NEN), multiple gland parathyroid disease, anterior pituitary adenomas and, less commonly, neuroendocrine tumors of the adrenal gland, bronchi and thymus [1, 2]. Pancreatic neuroendocrine neoplasms (pNEN), together with thymic tumors, represent the most common cause of death in MEN1 patients [3] if they are not identified and treated before malignant spread. It has been shown that the risk of aggressive behavior of pNENs increases significantly, if the tumor exceeds a size of 20 mm [4]. Therefore, it is essential for the management of MEN1 patients to diagnose and treat hormonally active NENs as well as NENs at risk for malignant behavior as early as possible.
Clinical practice guidelines recommend participation for all MEN1 patients in screening programs at expert centers [5, 6]. Regular screening should include at least laboratory tests as well as imaging for duodenopancreatic NENs [5]. There is no general recommendation for the type of imaging in routine screening of MEN1 patients, as clinical data are limited and imaging techniques depend on local conditions and preferences as well as resources.
The clinical use of the peptidic vector 1,4,7,10-tetraazacyclododecane-N,N′,N″,N‴-tetraacetic-acid-D-Phe1-Tyr3-octreotide (DOTATOC) was first published in 1997 [7]. It binds to somatostatin receptors (SSTR), which are expressed at high density in a majority of neuroendocrine tumors [8]. DOTATOC shows a high affinity for human SSTR 2 and SSTR 5 with a half-maximal inhibitory concentration of 14 nmol/l [9] and rapid renal clearance [10]. When labeled with the positron emitting nuclide Gallium-68, DOTATOC can be used for positron emission tomography (PET), which is superior to conventional SSTR-scintigraphy including detailed acquisition by single-photon emission tomography (SPECT) due to its higher spatial resolution [11]. Ga-68-DOTATOC-PET is combined with computed tomography (CT) or magnetic resonance imaging (MRI) for anatomical correlation of lesions. Ga-68-DOTATOC-PET-CT has proven to be a highly sensitive and specific technique for imaging of well-differentiated neuroendocrine tumors. The technique showed high sensitivity of 93–96% and specificity of 85–100% for sporadic NEN [12]. Ga-68-DOTATOC-PET-CT has also been found to be superior to conventional imaging (CT, MRI, ultrasonography) in the diagnosis and staging of sporadic neuroendocrine tumors [13, 14]. Disadvantages of this technique are the high costs and radiation exposure, if applied yearly.
While PET-CT using Ga-68-conjugated somatostatin analogues (DOTATOC, DOTA-TATE, DOTA-NOC) has been well assessed for sporadic NENs [12], only few case series have been reported on the use of Ga-68-DOTATOC-PET-CT in MEN1 patients [15–19]. Three of these four case series with 18, 19 and 26 MEN1-patients, respectively, compared Ga68-PET-CT to CT [15, 17, 18] and SRS/SPECT [15, 18] and found it to be superior to these techniques. One series presenting data from 18 MEN1 patients compared Ga-68-DOTATOC-PET-CT to EUS, CT of the trunk and MRI of the pituitary and found no benefit of the PET-CT compared to conventional imaging [16].
The aim of the present prospective study was to evaluate the value of Ga-68-DOTATOC-PET-CT in addition to conventional imaging in routine screening of MEN1 patients.
Patients and methods
Since 1997, the data of all MEN1 patients treated at the Department of Surgery of the University Hospital of Marburg have been documented in a prospective database after obtaining the patients informed consent in accordance with the local ethical committee. The database includes patients’ demographics, patients’ history, family history, underlying MEN1 mutations, laboratory and imaging findings and pathological findings after surgery.
The annual screening protocol included the measurement of laboratory parameters as well as imaging techniques as published previously by our group [20–22]. For the present study, conducted between January 2014 and March 2016, annual routine imaging was modified. In addition to contrast-enhanced MRI of the pituitary and abdomen and endoscopic ultrasonography (EUS), we also performed a contrast-enhanced CT of the thorax (instead of at 3 year intervals), an esophagogastroduodenoscopy (EGD) (instead of only in Zollinger–Ellison syndrome (ZES) patients or patients with specific gastroduodenal symptoms) and routine Ga-68-DOTATOC-PET-CT (instead of selected patients with specific characteristics) (Fig. 1). The measured laboratory values included plasma hormone levels of insulin, proinsulin, gastrin, pancreatic polypeptide, vasoactive intestinal peptide, glucagon, chromogranin A, serotonin, parathormone, prolactin and serum calcium. All MEN1 patients were recommended yearly screening unless previous findings required shorter surveillance periods. All diagnostic laboratory and imaging tests were performed within 48–72 h. Investigators were not blinded per protocol to each other’s results. However, since the written examination results were mostly available after 24–48 h, they were practically blinded for the vast majority of patients. The order of the examinations was not exactly defined per protocol and thus altered between patients.
Since this was an observational study, it was not registered as a clinical trial.
Endoscopic ultrasonography (EUS)
EUS was performed by an experienced endosonographer (PHK). Endosonography was carried out using a Pentax FG36UX endosonoscope (Pentax Corporation, Tokyo, Japan) with a longitudinal 5–7.5-MHz sector array in combination with a Hitachi EUB 525 ultrasound computer (Hitachi Medical Corporation, Tokyo, Japan). Premedication was performed with 50 mg pethidine, 5 – 30 mg diazepam and 0.125 – 0.5 mg atropine.
Magnetic resonance imaging (MRI)
MRI was performed using a 1.5 Tesla clinical MR scanner (Magnetom Sonata, Siemens, Erlangen, Germany) in one session. Axial T2-weighted as well as T1-weighted images with and without contrast agent (Magnevist, Bayer Schering Pharma, Berlin, Germany) were acquired. MRI images were reviewed by an experienced radiologist (JCA). Images were analyzed for focal lesions in the morphologic T1- and T2-weighted images. Lesions were measured in 2 dimensions and described according to number, shape and location.
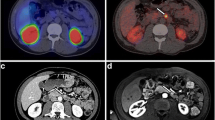
Ga-68-DOTATOC-PET-CT
Images were acquired within 45–60 min after tracer application using the PET-CT scanner Biograph 6 (Siemens, Forchheim, Germany). Acquisition area ranged from head to middle of the upper leg. Imaging data were normalized, and corrections were made for attenuation, dead time, decay and model-based scatter. The resulting images were evaluated visually by an experienced nuclear medicine physician (DL). Nonfocal linear intestinal uptake of moderate intensity as well as variable focal tracer uptake of pancreatic tissue (especially in the pancreatic head including the uncinate process) was considered physiological, while tracer accumulation in areas without physiological uptake or above background activity was classified as pathological. Tracer uptake clearly higher than liver uptake was considered positive for enhanced receptor expression and thus indicative of malignancy/positive lesions. Results of Ga-68-DOTATOC-PET-CT were assessed in collaboration of an experienced specialist for nuclear medicine (DL) and a radiologist (JCA).
Computed tomography of the thorax
CT scans were acquisitioned on a 64-row multislice detector (Siemens Somatom Definition, Siemens, Forchheim, Germany). Scan parameters were 1 mm slice thickness, 0.7 mm increment, soft tissue and lung tissue windowing with a reconstruction kernel of 30 and 70, 120 kV, weight-adapted mAs, axial, coronal and sagittal reconstructions. Images were reviewed by an experienced radiologist (JCA).
Statistical analysis
Differences in the median of two samples were analyzed by an unpaired t test for continuous normally distributed data. Mann–Whitney-U-test was used for nonparametric data. p values <0.05 were considered statistically significant. Data were analyzed using SPSS software (Version 16; SPSS, Inc., Chicago, IL). The data obtained were analyzed regarding changes in the diagnosis of manifestations of the syndrome as well as therapeutic management. A change of therapeutic management was defined by recommendation of shorter screening intervals or a change in medical or surgical treatment.
Results
Thirty-three genetically confirmed MEN1 patients (17 men and 16 women) with a median age of 44 (range 19–74) years underwent all screening modalities. Of these, 31 (94%) patients had previously detected GEP–NENs, including 6 (18%) patients with ZES, 2 (6%) patients with insulinoma and 31 (94%) patients with nonfunctioning pNENs (metachronous manifestations in 8 patients). Twenty-two (67%) patients had undergone GEP–NEN surgery, and 9 (27%) had malignant GEP–NEN disease. Five (15%) patients had previously diagnosed adrenal lesions, and 6 patients (18%) had bronchial NEN, whereas no patient had a known thymic NEN. Overall 7 (21%) patients had known lymph node and/or liver metastases. Clinical characteristics are summarized in Table 1.
Conventional imaging, including MRI, EUS, EGD and thoracic CT, detected 145 NEN in 27 (82%) of 33 patients, whereas Ga-68-DOTATOC-PET-CT detected only 55 somatostatin receptor positive lesions in 23 (70%) of 33 patients (p < 0.001). In 6 (26%) of these patients, no morphological correlate could be identified on the corresponding CT scan. The smallest NEN visualized by Ga-68-DOTATOC-PET-CT was 7 (range 7–50) mm in size, whereas conventional imaging, especially EUS, detected lesions as small as 2 (range 2–50) mm. The sensitivity of Ga-68-DOTATOC-PET-CT for NENs <5, 5–10, 10–19 and ≥20 mm was 0, 29, 81 and 100%, respectively, when using findings of all other imaging techniques as reference standard. Overall, 62% of NEN-typical lesions identified by conventional imaging were not detected by Ga-68-DOTATOC-PET-CT. Of the 55 Ga-68-DOTATOC-PET-CT positive lesions, 44 (80%) were located in the pancreas or duodenum. Apart from these, Ga-68-DOTATOC-PET-CT detected 11 lesions with increased tracer accumulation. These 11 lesions were found in 9 of 33 patients and included one adrenal adenoma, one gastric NEN, one NEN of the small intestine, 3 lymph node and 4 liver metastases and one pituitary adenoma. No bronchial or thymic NENs were detected by Ga-68-DOTATOC-PET-CT in the presented cohort (Table 2).
In the pancreas, the overall number of NENs detected by Ga-68-DOTATOC-PET-CT in 33 patients was 44 (median 1, range 0–5). MRI and EUS detected 113 pNENs (median 4, range 0–10). Ga-68-DOTATOC-PET-CT did not detect a pNEN that was not visible in EUS and/or MRI. Regarding the number and size of detected pNENs, MRI and EUS were superior to Ga-68-DOTATOC-PET-CT (p = 0.001, Table 3).
In 9 patients with a histologically proven malignant NEN disease, conventional imaging detected 2 new metastases (2 lymph nodes) compared to 7 new metastases (4 liver, 3 lymph nodes) detected by Ga-68-DOTATOC-PET-CT (p = 0.352). These 7 formerly unknown lesions were found in 4 patients. Three of these patients had known metastatic ZES, the fourth patient had a metastasized pituitary carcinoma. In only one of the patients with persistent ZES after Thompson’s procedure with suspected lymph node metastases on Ga-68-DOTATOC-PET-CT, no metastases could be found by conventional imaging. Two liver metastases detected in one patient could not be confirmed on a follow-up Ga-68-DOTATOC-PET-CT after a 12-month interval.
In two patients, a shortened screening period was recommended for pNEN sized 10–20 mm which showed increase in size on EUS.
The addition of Ga-68-DOTATOC-PET-CT to routine screening did not change the management in 97% (32/33) of patients. However, in one (3%) 39-year-old female patient with an SI–NEN, which had been missed by conventional imaging, the addition of Ga-68-DOTATOC-PET-CT resulted in potentially curative surgical resection of an UICC stage 3 tumor. The detection of additional metastases did not change the patients’ management in this series.
Discussion
Practice guidelines for the treatment of MEN1 patients recommend yearly routine screening. This includes clinical examination, laboratory testing and imaging [5], but the most effective imaging modalities are yet to be determined. The type of imaging, especially of the upper abdomen, has been debated, as there are controversial data on the sensitivity and specificity [23]. In addition, the type of imaging may be limited by given resources in certain centers and countries [5]. The role of Ga-68-DOTATOC-PET-CT is not discussed in the guidelines [5, 6] due to missing data and experience with this technique in the setting of MEN1. Four small-scale retrospective studies have been published on the use of Ga-68-DOTATOC-PET-CT in MEN1 screening [15–18]. All four studies proposed Ga-68-DOTATOC-PET-CT to be a valuable diagnostic tool in the setting of MEN1 in regard to sensitivity and specificity with the exception of parathyroid gland imaging [16]. These studies suggest that Ga-68-DOTATOC-PET-CT is superior to somatostatin receptor scintigraphy (SRS) and single-photon emission tomography (SPECT) and also superior to contrast-enhanced CT alone in the detection of NEN in MEN1 patients, which is in accordance with earlier data for sporadic NEN. However, only one small-scale study on 18 MEN1 patients provided detailed data comparing Ga-68-DOTATOC-PET-CT with multimodal MEN1 screening including laboratory testing, EUS, CT of the trunk and MRI of the pituitary [16]. The authors reported a sensitivity of up to 100% for the detection of NENs within the target organs except for the parathyroid glands and specificity of 83–100% when taking all other diagnostic techniques as reference standard. Even though Ga-68-DOTATOC-PET-CT did not reveal additional information, it was valued by the authors for giving a “panoramic view” over the manifestations of the syndrome.
The present study is the first to compare Ga-68-DOTATOC-PET-CT with conventional techniques for the complete screening of MEN1 patients. In a quite large cohort of 33 MEN1 patients with complete laboratory work-up and complete imaging including MRI of the abdomen, EUS, EGD, CT of the thorax and MRI, Ga-68-DOTATOC-PET-CT failed to visualize 92 of 145 NENs detected by other imaging modalities resulting in an overall sensitivity of only 37%. Analysis of the most common target organs excluding the parathyroid glands revealed a sensitivity for pancreatic NEN, adrenal and pituitary adenomas of 38, 20 and 7%, respectively. This low detection rate is partially due to the high physiological uptake of radiolabeled DOTATOC in the pituitary, adrenal and posterior portion of the pancreatic head [24]. The lower detection rate compared to previous studies may be also caused by the less extensive conventional screening performed by other study groups who described smaller numbers of 3.2 [17], 2.9 [16] and 3.9 [15] NENs detected per patient compared to 4.4 in the present study. Furthermore, small pNENs (<10 mm) that are commonly present within the pancreas of MEN1 patients (prevalence approx. 60–80% [22, 25, 26]) are well visualized by EUS, but rarely visualized by Ga-68-DOTATOC-PET-CT. The most likely reason is that in these small tumors the number of SSTR is not high enough to cause significant tracer uptake. The detection rate of larger tumors (>20 mm) of the pancreas, that are associated with an increased risk of metastasis [4, 25], is high. However, these tumors are reliably visualized by conventional imaging techniques as well. EUS was the most sensitive diagnostic tool for pancreaticoduodenal and adrenal lesions as well as lymph nodes in the upper retroperitoneum and hepatoduodenal ligament. Other imaging techniques failed to visualize 3 of 23 potentially meaningful pNENs sized 10–20 mm. Thus, EUS should not be omitted to ensure sensitive screening of the pancreas. Ga-68-DOTATOC-PET-CT has no additional benefit for the detection of NEN within the common target organs of MEN1.
The presented data, however, suggest that Ga-68-DOTATOC-PET-CT might be beneficial for imaging of metastatic disease. Although not statistically significant (p = 0.234), Ga-68-DOTATOC-PET-CT detected 7 new metastases (4 liver and 3 lymph node metastases) in 9 patients with a histologically proven malignant NET disease compared to only 2 new metastases (2 lymph nodes) in conventional imaging. However, the clinical management was not changed by the findings. These findings are partially in contrast to the study of 26 MEN1 patients by Sadowski et al. [15]. In that retrospective study, Ga-68-DOTATOC-PET-CT detected additional metastases in 10 patients (38.5%) compared to only SRS and CT of the abdomen but not MRI and EUS that changed the management of 8 (31%) patients. The difference to the presented study is not only because of EUS findings, but larger, therapy requiring NENs are reliably detected by MRI as well. However, in one of the patients of the present study Ga-68-DOTATOC-PET-CT detected an NEN of the small intestine that was not identified by conventional imaging and resulted in a potentially curative resection. In our institution, Ga68-DOTATOC-PET-CT will be performed only in MEN1 patients with known or suspected metastatic disease.
Conclusion
The routine use of Ga-68-DOTATOC-PET-CT for screening of asymptomatic MEN1 patients does not seem justified. It may be of additional use for the detection of neuroendocrine tumor tissue outside the common target organs, especially if metastatic disease is suspected.
References
Wermer P (1954) Genetic aspects of adenomatosis of endocrine glands. Am J Med 16:363–371
Trump D, Farren B, Wooding C et al (1996) Clinical studies of multiple endocrine neoplasia type 1 (MEN1). QJM 89:653–669
Goudet P, Murat A, Binquet C et al (2010) Risk factors and causes of death in MEN1 disease. A GTE (Groupe d’Etude des Tumeurs Endocrines) cohort study among 758 patients. World J Surg 34:249–255. doi:10.1007/s00268-009-0290-1
Triponez F, Dosseh D, Goudet P et al (2006) Epidemiology data on 108 MEN 1 patients from the GTE with isolated nonfunctioning tumors of the pancreas. Ann Surg 243:265–272
Thakker RV, Newey PJ, Walls GV et al (2012) Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J Clin Endocrinol Metab 97:2990–3011
Vinik AI, Woltering EA, Warner RR et al (2010) NANETS consensus guidelines for the diagnosis of neuroendocrine tumors. Pancreas 39:713–734
Otte A, Jermann E, Behe M et al (1997) DOTATOC: a powerful new tool for receptor-mediated radionuclide therapy. Eur J Nucl Med 24:792–795
van Essen M, Sundin A, Krenning EP et al (2014) Neuroendocrine tumours: the role of imaging for diagnosis and therapy. Nat Rev Endocrinol 10:102–114
Reubi JC, Schar JC, Waser B et al (2000) Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur J Nucl Med 27:273–282
Heppeler A, Froidevaux S, Macke HR et al (1999) Radiometal-labelled macrocyclic chelator-derivatised somatostatin analogue with superb tumour-targeting properties and potential for receptor-mediated internal radiotherapy. Chem Eur J 5:1974–1981
Henze M, Schuhmacher J, Hipp P et al (2001) PET imaging of somatostatin receptors using [68GA]DOTA-D-Phe1-Tyr3-octreotide: first results in patients with meningiomas. J Nucl Med 42:1053–1056
Yang J, Kan Y, Ge BH et al (2014) Diagnostic role of Gallium-68 DOTATOC and Gallium-68 DOTATATE PET in patients with neuroendocrine tumors: a meta-analysis. Acta Radiol 55:389–398
Ambrosini V, Campana D, Bodei L et al (2010) 68 Ga-DOTANOC PET/CT clinical impact in patients with neuroendocrine tumors. J Nucl Med 51:669–673
Sundin A, Garske U, Orlefors H (2007) Nuclear imaging of neuroendocrine tumours. Best Pract Res Clin Endocrinol Metab 21:69–85
Sadowski SM, Millo C, Cottle-Delisle C et al (2015) Results of (68)Gallium-DOTATATE PET/CT scanning in patients with multiple endocrine neoplasia Type 1. J Am Coll Surg 221:509–517
Lastoria S, Marciello F, Faggiano A et al (2016) Role of Ga-DOTATATE PET/CT in patients with multiple endocrine neoplasia type 1 (MEN1). Endocrine 52:488–494
Froeling V, Elgeti F, Maurer MH et al (2012) Impact of Ga-68 DOTATOC PET/CT on the diagnosis and treatment of patients with multiple endocrine neoplasia. Ann Nucl Med 26:738–743
Morgat C, Velayoudom-Cephise FL, Schwartz P et al (2016) Evaluation of Ga-DOTA-TOC PET/CT for the detection of duodenopancreatic neuroendocrine tumors in patients with MEN1. Eur J Nucl Med Mol Imaging 43:1258–1266
Sharma P, Mukherjee A, Karunanithi S et al (2015) Accuracy of 68 Ga DOTANOC PET/CT imaging in patients with multiple endocrine neoplasia syndromes. Clin Nucl Med 40:351–356
Lopez CL, Waldmann J, Fendrich V et al (2011) Long-term results of surgery for pancreatic neuroendocrine neoplasms in patients with MEN1. Langenbecks Arch Surg 396:1187–1196
Waldmann J, Fendrich V, Habbe N et al (2009) Screening of patients with multiple endocrine neoplasia type 1 (MEN-1): a critical analysis of its value. World J Surg 33:1208–1218. doi:10.1007/s00268-009-9983-8
Kann PH, Kann B, Fassbender WJ et al (2006) Small neuroendocrine pancreatic tumors in multiple endocrine neoplasia type 1 (MEN1): least significant change of tumor diameter as determined by endoscopic ultrasound (EUS) imaging. Exp Clin Endocrinol Diabetes 114:361–365
Ito T, Jensen RT (2016) Imaging in multiple endocrine neoplasia type 1: recent studies show enhanced sensitivities but increased controversies. Int J Endocr Oncol 3:53–66
Kroiss A, Putzer D, Decristoforo C et al (2013) 68 Ga-DOTA-TOC uptake in neuroendocrine tumour and healthy tissue: differentiation of physiological uptake and pathological processes in PET/CT. Eur J Nucl Med Mol Imaging 40:514–523
Partelli S, Tamburrino D, Lopez C et al (2016) Active surveillance versus surgery of nonfunctioning pancreatic neuroendocrine neoplasms ≤2 cm in MEN1 patients. Neuroendocrinology 103:779–786
Scarsbrook AF, Thakker RV, Wass JA et al (2006) Multiple endocrine neoplasia: spectrum of radiologic appearances and discussion of a multitechnique imaging approach. Radiographics 26:433–451
Acknowledgements
We thank all patients who participated in the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors state no conflicts of interest.
Rights and permissions
About this article
Cite this article
Albers, M.B., Librizzi, D., Lopez, C.L. et al. Limited Value of Ga-68-DOTATOC-PET-CT in Routine Screening of Patients with Multiple Endocrine Neoplasia Type 1. World J Surg 41, 1521–1527 (2017). https://doi.org/10.1007/s00268-017-3907-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-017-3907-9