Abstract
Background
Patients with persistent symptoms of acute cholecystitis for >72 h who cannot undergo urgent laparoscopic cholecystectomy (LC) often undergo percutaneous transhepatic gallbladder drainage (PTGBD) and delayed LC. However, intraoperative near-infrared fluorescence with indocyanine green (ICG) has recently become available in various surgical settings. Therefore, we evaluated the usability of intraoperative fluorescence imaging with ICG for LC after PTGBD in patients with acute cholecystitis.
Methods
The preoperative and postoperative clinical characteristics of patients who underwent LC after PTGBD were retrospectively analyzed.
Results
In total, 130 patients were reviewed. Intraoperative ICG fluorescence imaging was used in 39 (30.0%) patients, and none developed adverse reactions. Patients with ICG fluorescence imaging had a significantly shorter operative time (129 ± 46 vs. 150 ± 56 min, p = 0.0455), markedly lower conversion rate (2.6% vs. 22.0%, p = 0.0017), and lower proportion of subtotal cholecystectomy (0.0% vs. 6.6%, p = 0.0359) than patients without ICG fluorescence imaging. Independent risk factors for conversion to laparotomy during LC after PTGBD were the performance of PTGBD after 48 h from onset (OR 3.52; 95% CI 1.11–12.21; p = 0.0322), an unremoved PTGBD tube on LC (4.48, 1.46–15.00, p = 0.0084), and surgery without ICG (8.00, 1.28–159.47, p = 0.0231).
Conclusion
Intraoperative ICG fluorescence imaging produced better surgical outcomes without any adverse reactions. Early performance of PTGBD and intraoperative ICG fluorescence imaging can reduce the surgical difficulties in LC after PTGBD for acute cholecystitis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Laparoscopic cholecystectomy (LC) is one of the most commonly performed elective laparoscopic procedures. Iatrogenic bile duct injury (BDI) occurs infrequently (incidence of about 0.4%) but still represents an important complication [1,2,3]. Misinterpretation of the biliary anatomy because of unrecognized anatomical variations or an inflammatory status is the most frequent etiology of iatrogenic biliary tree lesions [4], and the rate of BDI can increase in the presence of cholecystitis [5].
Percutaneous transhepatic gallbladder drainage (PTGBD) is traditionally considered a safe alternative to early cholecystectomy in surgically high-risk patients with acute cholecystitis [6]. Some recent studies have indicated the safety and acceptability of percutaneous transhepatic aspiration [7] and the clinical efficacy of endoscopic gallbladder drainage [8]. The Tokyo Guidelines 2013 recommended early LC as the first-line treatment in patients with grade I and II acute cholecystitis; however, percutaneous and endoscopic gallbladder drainage is the first-line treatment in patients with grade III acute cholecystitis, some patients with grade II acute cholecystitis, and patients in whom early LC is difficult to perform [9]. Therefore, patients who require PTGBD are expected to have a higher severity grade and more severe inflammatory changes around the gallbladder than patients who do not require PTGBD. Severe inflammation of the gallbladder and its surroundings is known to be associated with an increased complication rate [10].
Intraoperative near-infrared fluorescence with indocyanine green (ICG) has recently become available in various surgical settings, such as breast surgery [11], gastrointestinal surgery [12], and hepato-biliary-pancreatic surgery [13,14,15]. Ishizawa T et al reported the first LC case with ICG fluorescence images [16]. The ICG fluorescence imaging technique is based on the principle that protein-bound ICG emits light with a peak wavelength of approximately 800 nm when illuminated with near-infrared light [17]. Although several reports have described intraoperative fluorescence imaging with ICG in LC [4, 18, 19], they reported the safety, feasibility, and efficacy in patients with a normal gallbladder. To our knowledge, no report has described the usability of intraoperative ICG fluorescence imaging in patients with severe inflammation after acute cholecystitis. Therefore, the aim of this study was to elucidate the usability of intraoperative fluorescence imaging with ICG for LC after PTGBD in patients with acute cholecystitis.
Methods
We reviewed patients who underwent elective LC after PTGBD for acute cholecystitis from January 2012 to December 2016 at Iizuka Hospital (Fukuoka, Japan). All acute cholecystitis patients were treated firstly in internal medicine department and later visited our department. Also, we introduced LC with ICG fluorescence images in July 2015, since then all LCs were performed with fluorescence cholangiography. This study was approved by an institutional review board (17141).
Intraoperative fluorescence imaging with ICG
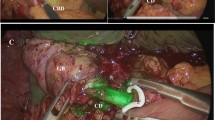
In all patients who underwent ICG fluorescence imaging, 2.5 mg of ICG (Diagnogreen®; Daiichi Sankyo Co., Ltd., Tokyo, Japan) was injected intravenously immediately after the induction of anesthesia to obtain intraoperative fluorescence illumination of the extrahepatic bile ducts (Fig. 1a). First, ICG fluorescence imaging was performed in about 30th min. To detect the cystic artery and right hepatic artery, a repeat intravenous injection of 2.5 mg of ICG was performed during the dissection of Calot’s triangle (Fig. 1b, c). In all cases, a laparoscopic fluorescence imaging system (Karl Storz GmbH & Co. KG, Tuttlingen, Germany), including a plasma light guide and 30°, 10-mm laparoscope applicable for white light, autofluorescence, and ICG imaging, was used for intraoperative conventional imaging and near-infrared fluorescence imaging (ICG mode). Visualization of both imaging is improved by a professional image enhancement system (IMAGE 1 SPIES system™; Karl Storz), which offers adjustable visualization modalities that can be selected according to the surgeon’s preference. The ICG mode was repeatedly used as needed during the operation. In this study, all patients underwent intravenous injection of ICG and none was performed ICG fluorescence imaging with intrabiliary injection.
a Intraoperative ICG fluorescence imaging during dissection of Calot’s triangle. The liver and extrahepatic bile ducts are illuminated. The white arrowheads indicate the cystic duct, and the black arrowheads indicate the common bile duct. b, c White light and ICG fluorescence image in a later stage of dissection of Calot’s triangle. Additional intravenous injection of ICG reveals the course of the cystic artery and right hepatic artery (right hepatic artery of this patient was ventral side of the common hepatic duct). The white arrowheads indicate the right hepatic artery, and the black arrowheads indicate the cystic artery in the white light image and ICG fluorescence image, respectively
Surgical procedures
Surgical procedures for LC were performed according to the critical view of safety technique described by Strasberg et al. [20], which entails visualization of Calot’s triangle. Surgeons could choose the most appropriate surgical devices with or without laparoscopic coagulating shears and soft coagulation (VIO system; Erbe, Tübingen, Germany). The criteria for conversion to laparotomy were dependent upon each surgeon and included factors such as the presence of adhesion, inflammatory change, or bleeding.
Preoperative imaging examinations
All patients without an allergy to contrast agent underwent DIC-CT+CTA. On the other hand, patients with an allergy were performed MRCP.
The indication of PTGBD for acute cholecystitis patients
In our institute, all acute cholecystitis patients were treated according to the Tokyo guidelines 2013. Grade I and II patients were treated with antibiotics at fast, and non-responders for initial treatment were performed PTGBD. Grade III patients were performed PTGBD immediately.
PTGBD tube removal criterion
The criterion for PTGBD tube removal was the detection of the common bile duct with injection of contrast dye through the PTGBD tube. Patients with this radiological imaging sign underwent tube removal and were discharged from the hospital and later underwent elective LC. Patients without these findings continued PTGBD and underwent surgery as soon as possible.
Statistical analysis
All statistical analyses were performed using SAS software (JMP 11.0.0; SAS Institute Inc., Cary, NC, USA). All variables are expressed as mean ± standard deviation. Categorical variables were compared using the chi-squared test, and continuous variables were compared using the nonparametric Wilcoxon test or the parametric t-test. Logistic regression analyses were performed to identify independent risk factors. A p value of <0.050 was considered statistically significant.
Results
Patients
A total of 746 patients underwent LC from January 2012 to December 2016 in our institute. Patients without cholecystitis (n = 395) and those who underwent urgent LC or did not undergo PTGBD for acute cholecystitis (n = 221) were excluded. The remaining 130 patients were enrolled in this study.
The patients comprised of 83 men and 47 women with a mean age of 68.6 ± 10.7 years. Eighteen (13.8%) patients had a history of cholecystitis, and 32 (24.6%) had a history of laparotomy. Ninety (69.2%) patients had grade II or III cholecystitis [21]. The duration from onset to surgery and to performance of PTGBD was 34.8 ± 23.7 and 2.9 ± 3.2 days, respectively. We used intraoperative fluorescence imaging with ICG in 39 (30.0%) patients, none of whom developed adverse reactions.
Comparisons between patients with and without intraoperative fluorescence imaging with ICG
Univariate analyses showed that patients who underwent intraoperative fluorescence imaging with ICG (ICG group, n = 39) had a significantly higher preoperative C-reactive protein concentration (2.99 ± 6.53 vs. 1.12 ± 2.53 mg/dL, p = 0.0206), longer duration from onset to surgery (42.7 ± 30.8 vs. 31.4 ± 19.1 days, p = 0.0121), and lower proportion of an unremoved PTGBD tube on LC (18.0% vs. 37.4%, p = 0.0241) than did the non-ICG group (n = 91) (Table 1). There were no significant differences in the history of cholecystitis or laparotomy, cholecystitis severity, preoperative and maximum white blood cell counts, or C-reactive protein concentration during the disease course. The surgical outcomes associated with the use of ICG imaging are shown in Table 2. The ICG group had a significantly shorter operative time (129 ± 46 vs. 150 ± 56 min, p = 0.0455), markedly lower conversion rate (2.6% vs. 22.0%, p = 0.0017), and lower proportion of subtotal cholecystectomy (0.0% vs. 6.6%, p = 0.0359) than the non-ICG group. There were no significant differences in the intraoperative blood loss volume or postoperative length of hospital stay. In particular, the rate of surgery-related postoperative complications was about 10% in both groups without a significant difference, and no ICG-related adverse reactions occurred in the ICG group.
Indications for conversion to laparotomy
Twenty patients underwent conversion in the non-ICG group. The most common reason for abandonment of LC was severe fibrosis and scarring in Calot’s triangle area or the gallbladder bed due to inflammation (n = 16). Two cases of BDI and two cases of cystic artery injury occurred. In contrast, only one patient underwent conversion in the ICG group, and this was due to bleeding from a peripheral branch of the middle hepatic vein in the gallbladder bed.
Risk factors for conversion to laparotomy during LC after PTGBD
To reveal the risk factors for conversion to laparotomy during LC after PTGBD, we compared two groups of patients: those who underwent conversion (n = 21) and those who only underwent laparoscopy (n = 109). Univariate analyses showed that the conversion group had a significantly higher proportion of moderate-to-severe cholecystitis (90.5% vs. 65.1%, p = 0.0359), longer duration from onset to performance of PTGBD (4.5 ± 4.9 vs. 2.6 ± 2.6 days, p = 0.0101), higher rate of performing PTGBD after 48 h from onset (66.7% vs. 31.2%, p = 0.0024), higher proportion of an unremoved PTGBD tube on LC (61.9% vs. 25.7%, p = 0.0016), and higher rate of LC without ICG fluorescence imaging (95.2% vs. 65.1%, p = 0.0017) (Table 3). Multivariate analyses were performed to identify independent risk factors for conversion to laparotomy (Table 4). These risk factors were the performance of PTGBD after 48 h from onset (OR 3.52; 95% CI 1.11–12.21; p = 0.0322), an unremoved PTGBD tube on LC (4.48, 1.46–15.00, p = 0.0084), and surgery without ICG (8.00, 1.28–159.47, p = 0.0231).
Discussion
LC has long been the first-line therapy for cholecystitis, and various studies have clarified the predictive factors for surgical difficulties and indications for conversion to laparotomy during LC for cholecystitis. According to the literature, these indications include iatrogenic BDI, severe fibrosis and scarring in Calot’s triangle area or the gallbladder bed due to inflammation, and bleeding [5, 10]. The critical view of safety technique, which was described by Strasberg et al. [20], is the standard approach with which to avoid iatrogenic BDI in LC. Additional intraoperative cholangiography was reported to significantly decrease the occurrence of BDI [1], and intraoperative fluorescence imaging with ICG was recently shown to be an alternative to intraoperative cholangiography for visualizing the extrahepatic biliary structures during LC [18]. To our knowledge, no study has been carried out to analyze the usability of intraoperative ICG fluorescence imaging to assess BDI in LC after PTGBD.
No BDI occurred in the ICG group of the present study because the initial ICG imaging allowed for recognition of the biliary tract before dissection of Calot’s triangle and repetitive real-time ICG imaging enabled the surgeons to avoid misidentifying the common bile duct or accessory hepatic duct as the cystic duct and to perform dissection without an inherent risk of BDI, especially on the right side of the common bile duct, before establishment of the critical view of safety. Moreover, obtaining multiple fluorescence images was extremely useful in patients with severe fibrosis and scarring around the gallbladder. Although those changes might make interpretation of the anatomy difficult, induce BDI or vascular injury, and generate a sense of anxiety in surgeons treating patients with BDI or vascular injury, real-time ICG images can overcome these problems. Even when the wall thickness of the biliary tract cannot be visualized because of cholecystitis, meticulous partial dissection can allow for biliary tract visualization [4, 18]. This indicates that non-fluorescing sites are not the biliary tract itself and can thus be safely dissected. Moreover, in the present study, additional ICG injection enabled surgeons to recognize the course of the cystic artery and right hepatic artery (Fig. 1b, c). Recognition of the arterial anatomy led to cautious dissection in patients with severe inflammation and reduced the risk of vascular injury. Therefore, intraoperative fluorescence with ICG in the current study reduced the indications for conversion to laparotomy and resulted in a conversion rate ranging from 2.6% to 22.0% and a proportion of subtotal cholecystectomy ranging from 0.0% to 6.6%. Liu et al. [4] reported that intra-gallbladder ICG injection was more useful for the visualization of the biliary tree than was conventional intravenous injection. Thus, the combination of intravenous and intra-gallbladder ICG injection might shorten the operative time in patients with an unremoved PTGBD tube undergoing LC.
PTGBD is traditionally considered a safe and effective procedure for acute cholecystitis, and the Tokyo Guidelines 2013 recommend it as the standard gallbladder drainage method in critically ill patients or patients who cannot undergo urgent LC [21]. Several studies have demonstrated various surgical difficulties in LC after PTGBD, including a higher rate of conversion to laparotomy, longer operation time, and more intraoperative blood loss than in patients without PTGBD [6, 22, 23]. After publication of the latest guidelines, delayed LC after PTGBD was reported to produce better outcomes than emergent LC [24, 25]. In their prospective study, El-Gendi et al. [24] found that better outcomes were obtained when LC was delayed 6 weeks after PTGBD. In the present study, however, the duration from the performance of PTGBD to surgery was not significantly different between the two groups (p = 0.4648). Although no prospective study has analyzed the duration from onset to performance of PTGBD, Yamada et al. [6] performed such an analysis in their retrospective study. They found that a longer interval could regress inflammatory adhesion and might induce better surgical outcomes (shorter operative time, less intraoperative blood loss, and lower rate of conversion). As in the current study, they also concluded that the early performance of PTGBD induces good surgical outcomes. Interestingly, they studied the pathologic findings after PTGBD for acute cholecystitis and showed that the early performance of PTGBD resulted in a high rate of edematous cholecystitis, consequently reducing surgical difficulties. They assumed that PTGBD might stop the progression of cholecystitis and prevent inflammation from spreading to the surrounding tissue. Therefore, it is most important to perform PTGBD earlier as possible in case of not performing urgent LC.
The main limitation of this study is its retrospective nature, which limited the data available for analysis. This study also included a relatively small number of inhomogeneous patients. Although various surgeons performed LC in this study, the analyses did not account for potential differences in the background characteristics among the surgeons who decided to perform conversion to laparotomy because of severe inflammatory changes. Further prospective analysis is necessary to confirm our findings.
Conclusion
Intraoperative ICG fluorescence imaging produced better surgical outcomes with a shorter operative time, markedly lower rate of conversion to laparotomy, and lower rate of subtotal cholecystectomy without any adverse reactions. These findings indicate that the early performance of PTGBD and intraoperative ICG fluorescence imaging can reduce the surgical difficulties in LC after PTGBD for acute cholecystitis.
Abbreviations
- BDI:
-
Bile duct injury
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- CRP:
-
C-reactive protein
- CTA:
-
CT angiography
- DIC-CT:
-
Drip infusion cholecystocholangiography-CT
- ICG:
-
Indocyanine green
- LC:
-
Laparoscopic cholecystectomy
- PTGBD:
-
Percutaneous transhepatic gallbladder drainage
- WBC:
-
White blood cell
References
Flum DR, Dellinger EP, Cheadle A et al (2003) Intraoperative cholangiography and risk of common bile duct injury during cholecystectomy. JAMA J Am Med Assoc 289:1639–1644
Nuzzo G, Giuliante F, Giovannini I et al (2005) Bile duct injury during laparoscopic cholecystectomy: results of an Italian national survey on 56 591 cholecystectomies. Arch Surg 140:986–992
Waage A, Nilsson M (2006) Iatrogenic bile duct injury: a population-based study of 152 776 cholecystectomies in the Swedish Inpatient Registry. Arch Surg 141:1207–1213
Liu YY, Kong SH, Diana M et al (2016) Near-infrared cholecysto-cholangiography with indocyanine green may secure cholecystectomy in difficult clinical situations: proof of the concept in a porcine model. Surg Endosc 30:4115–4123
Navez B, Ungureanu F, Michiels M et al (2012) Surgical management of acute cholecystitis: results of a 2-year prospective multicenter survey in Belgium. Surg Endosc 26:2436–2445
Yamada K, Yamashita Y, Yamada T et al (2015) Optimal timing for performing percutaneous transhepatic gallbladder drainage and subsequent cholecystectomy for better management of acute cholecystitis. J Hepato-Biliary-Pancreat Sci 22:855–861
Komatsu S, Tsukamoto T, Iwasaki T et al (2014) Role of percutaneous transhepatic gallbladder aspiration in the early management of acute cholecystitis. J Dig Dis 15:669–675
Itoi T, Takada T, Hwang TL et al (2017) Percutaneous and endoscopic gallbladder drainage for acute cholecystitis: international multicenter comparative study using propensity score-matched analysis. J Hepato-Biliary-Pancreat Sci 24:362–368
Miura F, Takada T, Strasberg SM et al (2013) TG13 flowchart for the management of acute cholangitis and cholecystitis. J Hepato-Biliary-Pancreat Sci 20:47–54
Iwashita Y, Ohyama T, Honda G et al (2016) What are the appropriate indicators of surgical difficulty during laparoscopic cholecystectomy? Results from a Japan-Korea-Taiwan multinational survey. J Hepato-Biliary-Pancreat Sci 23:533–547
Sugie T, Ikeda T, Kawaguchi A et al (2017) Sentinel lymph node biopsy using indocyanine green fluorescence in early-stage breast cancer: a meta-analysis. Int J Clin Oncol 22:11–17
Yukaya T, Saeki H, Kasagi Y et al (2015) Indocyanine green fluorescence angiography for quantitative evaluation of gastric tube perfusion in patients undergoing esophagectomy. J Am Coll Surg 221:e37–e42
Kawaguchi Y, Nomura Y, Nagai M et al (2017) Liver transection using indocyanine green fluorescence imaging and hepatic vein clamping. Br J Surg 104:898–906
Mitsuhashi N, Kimura F, Shimizu H et al (2008) Usefulness of intraoperative fluorescence imaging to evaluate local anatomy in hepatobiliary surgery. J Hepato-Biliary-Pancreat Surg 15:508–514
Ishizawa T, Tamura S, Masuda K et al (2009) Intraoperative fluorescent cholangiography using indocyanine green: a biliary road map for safe surgery. J Am Coll Surg 208:e1–e4
Ishizawa T, Bandai Y, Kokudo N (2009) Fluorescent cholangiography using indocyanine green for laparoscopic cholecystectomy: an initial experience. Arch Surg 144:381–382
Mordon S, Devoisselle JM, Soulie-Begu S et al (1998) Indocyanine green: physicochemical factors affecting its fluorescence in vivo. Microvasc Res 55:146–152
Osayi SN, Wendling MR, Drosdeck JM et al (2015) Near-infrared fluorescent cholangiography facilitates identification of biliary anatomy during laparoscopic cholecystectomy. Surg Endosc 29:368–375
Ishizawa T, Bandai Y, Ijichi M et al (2010) Fluorescent cholangiography illuminating the biliary tree during laparoscopic cholecystectomy. Br J Surg 97:1369–1377
Strasberg SM, Hertl M, Soper NJ (1995) An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg 180:101–125
Takada T, Strasberg SM, Solomkin JS et al (2013) TG13: Updated Tokyo Guidelines for the management of acute cholangitis and cholecystitis. J Hepato-Biliary-Pancreat Sci 20:1–7
de Mestral C, Gomez D, Haas B et al (2013) Cholecystostomy: a bridge to hospital discharge but not delayed cholecystectomy. J Trauma Acute Care Surg 74:175–179 discussion 179-180
Paran H, Zissin R, Rosenberg E et al (2006) Prospective evaluation of patients with acute cholecystitis treated with percutaneous cholecystostomy and interval laparoscopic cholecystectomy. Int J Surg 4:101–105
El-Gendi A, El-Shafei M, Emara D (2017) Emergency versus delayed cholecystectomy after percutaneous transhepatic gallbladder drainage in grade II acute cholecystitis patients. J Gastrointest Surg 21:284–293
Shibasaki S, Takahashi N, Toi H et al (2014) Percutaneous transhepatic gallbladder drainage followed by elective laparoscopic cholecystectomy in patients with moderate acute cholecystitis under antithrombotic therapy. J Hepato-Biliary-Pancreat Sci 21:335–342
Acknowledgements
This study was supported in part by a Grant-in-Aid for Scientific Research (KAKENHI) from the Ministry of Health, Labour and Welfare of Japan (16K19935). The funding source had no role in the collection, analysis, or interpretation of the data, or in the decision to submit the article for publication. The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoshiya, S., Minagawa, R., Kamo, K. et al. Usability of Intraoperative Fluorescence Imaging with Indocyanine Green During Laparoscopic Cholecystectomy After Percutaneous Transhepatic Gallbladder Drainage. World J Surg 43, 127–133 (2019). https://doi.org/10.1007/s00268-018-4760-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-018-4760-1