Abstract
Purpose
To establish the optimal dose of indocyanine green (ICG) to administer intravenously 30 min before laparoscopic cholecystectomy (LC).
Methods
In this randomized controlled trial (RCT), patients undergoing LC for cholecystitis, cholelithiasis, and/or cholecystic polyps were randomized into four groups given four different ICG doses (0.025, 0.1, 0.25, 2.5 mg). Using OptoMedic endoscopy combined with a near-infrared fluorescent imaging system, we evaluated the fluorescence intensity (FI) of the common bile duct and liver at three timepoints: before surgical dissection of the cystohepatic triangle, before clipping of the cystic duct, and before closure. The bile duct-to-liver ratio (BLR) of the FI was analyzed to assess the cholangiography effect.
Results
Sixty-four patients were allocated to one of four groups, with 40 patients included in the final analysis. Generally, with increasing ICG doses, the levels of FI in the bile duct and liver increased gradually at each of the three timepoints. Before surgical dissection of the cystohepatic triangle, 0.1-mg ICG showed the highest BLR (F = 3.47, p = 0.0259). Before clipping the cystic duct and before closure, the 0.025- and 0.1-mg groups showed a higher BLR than the 0.25- and 2.5-mg groups (p < 0.05). When setting the ideal cholangiography at a BLR ≥ 1, ≥ 3, or ≥ 5, the 0.1-mg group showed the highest qualified case number at the three timepoints.
Conclusions
The intravenous administration of 0.1-mg ICG, 30 min before LC, is significantly better for fluorescent cholangiography of the extrahepatic biliary structures before dissection and clipping of the cystohepatic triangle.
Trial registration
This study was registered in the Chinese Clinical Trial Registry (ChiCTR) (ChiCTR2200057933).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Laparoscopic cholecystectomy (LC) is a common surgical procedure for biliary diseases [1]. The incidence of bile duct injury (BDI) during LC is still relatively high, ranging between 0.2% and 1.5% [2,3,4]. As a surgical complication, BDI is related to mortality, increased costs, and compromised quality of life [1]. Strategies to decrease the incidence of BDI include initiatory anatomical identification skills, such as the infundibular technique [5, 6] and a critical view of safety [7,8,9], and intraoperative cholangiography (IOC) techniques, such as laparoscopic ultrasonography [10, 11], radiographic IOC [12, 13], and fluorescent IOC with indocyanine green (ICG) [13,14,15] injected via the intrabiliary or intravenous route [16]. Anatomical identification skills require years of clinical surgical experience, so they are not feasible for junior doctors. Radiographic IOC is not used widely in clinical settings because of limitations, such as X-ray exposure, prolonged operation time, and the need for more medical equipment and staff [12].
The fluorescent IOC of bile ducts using near-infrared (NIR) light and ICG during LC has been introduced [15]. The ICG fluorescent imaging system can show real-time direction of the extrahepatic biliary tract; thereby, playing a navigational role in hepatobiliary surgery. In a randomized controlled trial (RCT) [17], ICG fluorescence imaging was found to be superior to ordinary high-definition (HD) LC for intraoperative identification and cholangiography of the extrahepatic biliary tract [17]. However, the clinical application of fluorescent IOC with ICG is hampered by different ICG doses and injection times [18]. In our previous single-center trial [19], we optimized the ICG dose by injecting it 10 h before surgery. We showed that the optimal effect of fluorescent cholangiography was achieved by delivering a 10-mg ICG injection 10–12 h preoperatively [19]. However, injecting ICG 10 h before surgery is not always practical. For example, when consecutive operations are planned, we cannot estimate the preceding operation time, while some patients may unexpectedly decide to postpone their operation, in which case, the early injection of ICG would be pointless. Clinically, the intervening time within an hour or just after the induction of anesthesia would be most practical, and about 30 min before surgery is believed to be a good time interval with high controllability in our center.
In this RCT, we explored establishing a more practical ICG dose by injecting it intravenously within 30 min preoperatively. Our intravenous ICG injection time was 30 min before surgery, just after the patient’s anesthetization. Four different ICG doses (0.025, 0.1, 0.25, and 2.5 mg) were given during LC. Using an OptoMedic endoscope armed with an NIR fluorescent (NIRF) imaging system [19], we evaluated the fluorescence intensity (FI) of the common bile duct (CBD) and the liver. Technically, three timepoints (before surgical dissection of the cystohepatic triangle, before clipping of the cystic duct, and before closure) were evaluated in this trial. Finally, we analyzed the bile duct-to-liver ratio (BLR) of FI. NIRF with 0.1-mg ICG within 30 min preoperatively was significantly better for visualizing the extrahepatic biliary structures before dissection and clipping of the cystohepatic triangle during LC.
Materials and methods
Patients
All patients admitted to our department to undergo LC for cholecystitis, cholelithiasis, and/or cholecystic polyps were screened carefully for possible enrollment in this single-center RCT.
The inclusion criteria were as follows: a clear indication for LC surgery; a serum total bilirubin level less than 30 μmol/L; no signs of liver cirrhosis on computed tomography (CT) or ultrasound imaging; age ≥ 18 years; ability to understand and follow the study procedures; and signed informed consent. The exclusion criteria were as follows: an allergy to iodides or iodine dyes; an allergy to ICG; pregnancy or breast feeding; or being of reproductive age with the possibility of pregnancy.
Study design
This randomized, double-blind clinical trial was conducted at the Department of Surgery, Guangzhou First People’s Hospital. The patients were randomly allocated into four groups (A, B, C, D), to receive one of four doses of ICG (0.025, 0.1, 0.25, 2.5 mg) intravenously. This study, approved by the Research Ethics Committee of the Guangzhou First People’s Hospital (approval NO: k-2022–033-01), complied with the requirements of the Declaration of Helsinki. Informed consent was obtained from all of the recruited patients. This study was registered in the Chinese Clinical Trial Registry (ChiCTR) (ChiCTR2200057933).
Imaging system
Fluorescent images were collected using an HD fluorescence laparoscope equipped with an NIRF imaging system (2100 Series, Fig. S1A) offered by Guangdong OptoMedic Technologies Inc, Guangzhou, China (web link: http://www.optomedic.com). This HD brilliant imaging system was an OPTO-CAM2100 with a resolution of 1920 × 1080P and 60 frames/second. The light source was an OPTO-LED2100 with near-infrared light of a 30-degree endoscope. Four different imaging modalities; namely, standard white light mode, standard fluorescence mode, pseudo-color fluorescence mode, and multidisplay mode with grayscale image inside, could be interchanged during surgery (Fig. S1B).
Sample size
G*Power [20] was used to estimate the sample size. As four different groups were included in our trial, one-way ANOVA with F tests was used for statistical testing. For power analysis, we chose “a priori: computer required sample size by giving the α-error, power, and the effect size” (Fig. S2). In the input parameters box, we set the α-error at 0.05, the power (1 − β) at 0.95, and the effect size f at 0.7 (assuming a baseline bile duct detection rate of 70% with ICG, based on a previous study). The total required sample size of each of the four groups was calculated. Finally, 40 patients were needed in our trial, in which the critical F value was 2.866 and the actual power was 0.954 (Fig. S2). As we collected only the surgical data of fluorescent cholangiography images in LC, no follow-up was included in this study.
Randomization
The included patients were numbered serially from 1 to 40 according to the order of admission. The 40 were then divided randomly into four groups with the allocation ratio of 1:1:1:1, using a randomization sequence generated by SPSS 26.0. Based on the results, the patients were assigned randomly to groups A, B, C, and D. A double-blinded setting was used, as the patients were unaware of the grouping information and the ICG injection staff was unaware of the ICG doses. After a patient was accepted by the recruitment team and before the ICG injection, an appropriate numbered envelope with dose and group information inside was opened by a statistician. The card inside contained the patient’s assigned group (A, B, C, or D) and the ICG injection dose details. The collected results were summarized and analyzed by the statistician blinded to the grouping information and the ICG dose.
Intervention
ICG was administered intravenously 30 min before surgery, just after the induction of anesthesia. The ICG administration time and any adverse reactions were recorded. After inserting a trocar into the abdominal cavity and completing other standard operative procedures such as releasing adhesions, the florescent images and the precise time were recorded when the cystohepatic triangle was first seen. After dissecting the tissues surrounding the gallbladder to permit cholangiography of the cystic duct, common hepatic duct, and CBD, but before gall bladder resection and immediately before clipping, the surgeon recorded the florescent images and the operating time to clip point (time-to-clip). Finally, the surgeon used NIRF to obtain the florescent images and the precise time before closing, or the time for starting exploration of the CBD if necessary.
FI measurements
The FI of the CBD and the liver was measured using ImageJ. The greyscale images from the multidisplay mode were used for measuring the FI. The regions of interest (ROIs) in the CBD were chosen to be as large as possible in images with ICG areas (Fig. S3), and the ROIs in the liver background were chosen from the ICG areas in the liver (Fig. S3). On an average, the mean ROIs in the CBD ranged from 3000 to 6500, and the ROIs in the liver ranged from 6000 to 19,500. The BLR was calculated as follows: BLR = \(\frac{FI(bile duct)}{FI(liver)}\).
Statistical analysis
We examined whether all data were distributed normally. The data are presented as the mean ± standard deviation (SD) or as the number of patients. A two-sided p value of < 0.05 was considered statistically significant. For intergroup comparisons of continuous variables, an analysis of variance (ANOVA) was conducted. The Bonferroni method was used for pairwise comparisons between multiple groups to correct the significance level. Statistical analysis was performed using SPSS 26.0 and the figures were generated with GraphPad Prism 8.
Results
Patients
We assessed 64 patients undergoing LC at a single center for eligibility in this random controlled trial. After the exclusion of 24 patients who did not meet the inclusion criteria (n = 15), withdrew consent (n = 4), or could not be included for other reasons such as a shortage of instruments (n = 5), 40 patients were included in the final analysis. These 40 patients, with a balanced male-to-female ratio, were randomized to the four treatment groups (n = 10 each; Fig. 1). Table 1 summarizes the demographic and baseline clinical data of the four groups.
The patients were aged 57.4 ± 15.6 (57.7 ± 4.987 [Group A] vs. 51.6 ± 4.492 [Group B] vs. 64.4 ± 4.738 [Group C] vs. 55.7 ± 5.325 [Group D]). The mean body mass index (BMI) was 23.7 ± 3.5 kg/m2 (22.82 ± 0.733 [Group A] vs. 24.41 ± 1.220 [Group B] vs. 23.89 ± 1.132 [Group C] vs. 23.57 ± 1.353 [Group D]). Five patients underwent abdominal surgery, but none had evidence of liver cirrhosis on CT or ultrasound imaging. Most of the patients underwent LC, while six underwent LC with CBD exploration (LC + CBDE). There were no significant intergroup differences in age, gender ratio, BMI, postoperative hospitalization duration, operation time, blood loss, or preoperative levels of aspartate aminotransferase, alanine aminotransferase, total bilirubin, and direct bilirubin (Table 1).
Cholangiography
To explore the optimal cholangiography view during LC, we collected fluorescence intensity images of the CBD and liver at three timepoints: before surgical dissection of the cystohepatic triangle, before clipping of the cystic duct, and before closure. The time from operation to dissection of the cystohepatic triangle was 37.7 ± 12.3 min (34.43 ± 2.716 [Group A] vs. 38.50 ± 2.257 [Group B] vs. 33.57 ± 2.644 [Group C] vs. 41.20 ± 6.425 [Group D]); the time from operation to clipping of the cystic duct was 61.1 ± 23.2 min (52.29 ± 6.144 [Group A] vs. 55.40 ± 3.187 [Group B] vs. 73.71 ± 14.093 [Group C] vs. 65.00 ± 8.881 [Group D]); and the time from operation to closure was 79.9 ± 25.0 min (75.57 ± 8.237 [Group A] vs. 80.90 ± 5.956 [Group B] vs. 85.14 ± 12.889 [Group C] vs. 82.30 ± 10.323 [Group D]), without significant differences among the groups (Table 2).
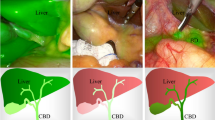
During surgical dissection of the cystohepatic triangle (Fig. 2A), 0.025-mg ICG injection (Group A) showed a small amount of green dye in the CBD. With increasing ICG doses (from groups A to D), the green dye in the CBD increased. When 0.25-mg ICG was injected (Group C), the liver background showed a relatively large amount of green dye, but this was most apparent in Group D, where the heavily dyed green liver background interfered with the cholangiography of the extrahepatic bile ducts. Before the cystic duct clipping, Groups A and B showed clear cholangiography of the CBD without a heavily dyed background in the liver (Fig. 2B). Groups C and D showed green dye in the CBD and a heavily dyed green liver background. These trends of ICG change in the bile ducts and liver background were also examined at the before-closure timepoint (Fig. 2C).
Representative images before dissection of the cystohepatic triangle, before clipping of the cystic duct, and before closure among the groups. A Fluorescent pictures and a greyscale image before dissection of the cystohepatic triangle; B a greyscale image before clipping of the cystic duct; C a greyscale image before closure. The white arrow shows the common bile duct
FI and BLR outcomes
On comparing the FI levels in the CBD and liver, we found that with increasing ICG doses from groups A to D, the FI levels in the bile duct and the liver background increased at the three timepoints (Fig. 3).
Fluorescent intensity levels in the bile duct and liver. Fluorescent intensity levels in the bile duct before surgical dissection of the cystohepatic triangle (A), before clipping of the cystic duct (B), and before closure (C). FI levels in the liver before surgical dissection of the cystohepatic triangle (D), before clipping of the cystic duct (E), and before closure (F). FI fluorescent intensity
BLR was introduced for a quantitative comparison of the surgical cholangiography by ICG. We analyzed the BLRs at the three timepoints. During the surgical dissection of the cystohepatic triangle, Group B showed a higher BLR than the other three groups (Fig. 4A). Before clipping the cystic duct, Groups A and B showed a relatively higher BLR than Groups C and D (Fig. 4B). Finally, at the before-closure timepoint, Groups A and B showed a relatively higher BLR than Groups C and D (Fig. 4C).
Bile-to-liver ratios. A Bile to liver ratio before surgical dissection of the cystohepatic triangle, B BLR before clipping of the cystic duct, C BLR before closure; D the case number for BLR ≥ 1, ≥ 3, and ≥ 5. The data were analyzed with ANOVA; The F value and p value are listed. BLR bile-to-liver ratio
We compared the number of patients with a BLR ≥ 1, ≥ 3, or ≥ 5 (Fig. 4D). When setting the BLR at ≥ 1, at the timepoint before dissection, there were seven, eight, three, and zero patients with a BLR ≥ 1 in Groups A, B, C, and D, respectively; at the timepoint before clipping, there were eight, nine, seven, and three patients with a BLR ≥ 1 in Groups A, B, C, and D, respectively. When setting the BLR at ≥ 3 before dissection, there was one patient with a BLR ≥ 3 in Group A, four in Group B, and none in Groups C and D. At the timepoint before clipping, there were five patients with a BLR ≥ 3 in Groups A and B, but none in Groups C and D. When setting the BLR ≥ 5 before dissection, there was one patient with a BLR ≥ 5 in Group A, four in Group B, and none in Groups C and D. At the other two timepoints, there were five patients with a BLR ≥ 5 in Groups A and B, but none in Groups C and D.
Discussion
Several factors affect cholangiography of the extrahepatic biliary tract using ICG, including the dose, time of injection, fluorescence imaging system, and the method of administration (intravenous or direct injection into the gallbladder) [21, 22]. The time of injection and dose are the two most important factors [21, 22]. Previous studies have used different ICG doses at different injection times. In a review of fluorescent cholangiography, Leonora et al. [18] stated that the ICG dose was usually 2.5 mg (55.6%), while other doses were 0.025, 5, 10, or 12.5 mg, or calculated according to body weight (0.05 mg/kg or 0.3–0.4 mg/mL/kg). The ICG injection time also varied. Among the 27 reported articles, the time of injection was “after intubation” in 33.3%; “during surgery” in 11.1%; within 1 h preoperatively, such as 15, 30, 45, or 60 min, in 51.8%; and even up to 24 h preoperatively. Hence, most studies administered 2.5-mg ICG, 1 h before imaging [18]. The injection method was generally peripheral intravenous injection and rarely a direct injection into the gallbladder. Moreover, various imaging systems were used. With the development of new imaging systems, such as HD or 4 K fluorescence endoscopy, the dose and time of ICG administration should be readjusted.
In this trial, we identified a more clinically practical time of injection: 30 min before surgery, which we believe is a good time interval with high controllability. In clinical activities for LC, nerve block anesthesia and urinary catheterization are usually set after intubation. Therefore, ICG administration could be performed between finishing intubation and nerve block anesthesia or urinary catheterization. In this way, we could set a 30-min interval from ICG injection to the beginning of surgery, including nerve block anesthesia (~ 5 min); urinary catheterization (~ 5 min); surgeon hand washing [3–5 min]; disinfection of the surgical area (~ 5 min); preparation of imaging system, camera sensor, and light source (~ 5 min); and the final check of the patient’s information before surgery [1–3 min].
We selected four different ICG doses (0.025, 0.1. 0.25, 2.5 mg) using OptoMedic endoscopes armed with the NIRF imaging system. Finally, we evaluated the FI of the CBD and liver at three timepoints: before surgical dissection of the cystohepatic triangle, before clipping the cystic duct, and before closure. Our data showed that with increasing ICG doses, the FI levels of the bile duct and liver increased gradually within the three timepoints.
The BLR is a useful index. Although the reported identification rates of common ducts were as high as > 90% in the literature [15, 23, 24], the BLR was barely satisfactory. Previous clinical trials demonstrated that the highest BLR was achieved 3–7 h after the injection of 5 mg ICG and 5–25 h after the injection of 10-mg ICG [18]. Up to 3 h after the injection of 5-mg ICG and up to 5 h after the injection of 10-mg ICG, the liver was equally or more fluorescent than the cystic duct, resulting in a ratio ≤ 1, meaning that the liver had a heavy background and weakened the cholangiography of the bile duct. In our trial, the injections of 0.025- and 0.1-mg ICG 30 min before surgery showed a relatively higher BLR before dissection, before clipping, and before closure, demonstrating good real-time fluorescent cholangiography during the whole procedure. In Group A, the BLRs ≥ 1 before dissection, before clipping, and before closure were 70%, 80%, and 90%, respectively. After the injection of 0.1-mg ICG (Group B), the BLRs ≥ 1 before dissection, before clipping, and before closure were 80%, 90%, and 90%, respectively. For Groups C and D, the BLR ≥ 1 before dissection was 30% and 0%, respectively, demonstrating that a relatively low ICG dose (0.025 and 0.1 mg in this study) provided good fluorescent cholangiography from the beginning of surgical dissection of the cystohepatic triangle.
Most studies regard a BLR ≥ 1 as ideal for bile-to-liver fluorescent cholangiography [18, 22, 25] based on the use of relatively high doses of ICG, such as 2.5, 5, and 10 mg, which require a long time to achieve the highest BLR. It takes 3–7 h after the administration of 5-mg ICG and 5–25 h after administration of 10-mg ICG to achieve the highest BLR [18]. Clinically, these times are impractical for patients undergoing a consecutive operation or for patients who may decide to suspend surgery. Uniquely, we set a BLR of ≥ 3 or ≥ 5 as ideal for bile-to-liver fluorescent cholangiography, as it presents a higher contrast ratio between the CBD and the liver than a BLR ≥ 1 in the same video, thereby offering much better fluorescent cholangiography to resident surgeons. In this context, the number of patients with a BLR ≥ 3 and ≥ 5 in our RCT were compared. We found a BLR ≥ 5 in one patient (10%) from Group A, four (40%) from Group B, and none from Groups C and D, at the predissection timepoint. At the before-clipping and before-closure timepoints, five patients (50%) from Groups A and B, but none from Groups C and D had a BLR ≥ 5. A similar changing trend was seen with a BLR ≥ 3 (Fig. 4D). We regard a BLR ≥ 5 as ideal for bile-to-liver fluorescence visualization because the higher the BLR is, the clearer the bile duct can be distinguished. Hence, 0.1-mg ICG injection 30 min before surgery was significantly better for visualizing extrahepatic biliary structures during the surgical procedure.
We included patients with normal liver function and no intrahepatic or extrahepatic biliary obstruction, and none had liver cirrhosis on CT or ultrasound imaging. However, many patients who undergo LC have intrahepatic or extrahepatic biliary obstruction or reduced liver function with cirrhosis. For those patients, biliary obstruction or impaired liver function would affect ICG distribution in the liver and its excretion to the bile duct, increasing the complexity of fluorescent cholangiography. Even so, our data may provide baseline data to apply the new technology to such complex situations.
This RCT examined the optimal dose of ICG applied within 30 min preoperatively, which is a more practical interval. The limitations of this trial include the small sample size and the use of the HD laparoscopy combined with an NIRF imaging system offered by Guangdong OptoMedic Technologies, China. Further studies with a larger sample size and using other imaging systems, such as 4 K systems, are needed to verify the optimal dose and injection time. In addition, the superiority of these settings should be compared with HD laparoscopes.
Conclusions
NIRF with 0.1-mg ICG, administered intravenously within 30 min preoperatively, was found to be significantly better for visualizing extrahepatic biliary structures before dissection and clipping of the cystohepatic triangle during LC. This allows for optimal identification and cholangiography of the extrahepatic biliary tract and plays a navigational role in hepatobiliary surgery.
Abbreviations
- NIRF:
-
Near-infrared fluorescence
- ICG:
-
Indocyanine green
- LC:
-
Laparoscopic cholecystectomy
- RCT:
-
Randomized controlled trial
- BLR:
-
Bile duct-to-liver ratio
- FI:
-
Fluorescence intensity
- BDI:
-
Bile duct injury
- IOC:
-
Intraoperative cholangiography
- HD:
-
High definition
- CT:
-
Computed tomography
- ANOVA:
-
Analysis of variance
- CBD:
-
Common bile duct
- CBDE:
-
Common bile duct exploration
- ROIs:
-
Regions of interest
References
Pucher PH, Brunt LM, Davies N, Linsk A, Munshi A, Rodriguez HA, et al. Outcome trends and safety measures after 30 years of laparoscopic cholecystectomy: a systematic review and pooled data analysis. Surg Endosc. 2018;32(5):2175–83.
Deziel DJ, Millikan KW, Economou SG, Doolas A, Ko ST, Airan MC. Complications of laparoscopic cholecystectomy: a national survey of 4292 hospitals and an analysis of 77,604 cases. Am J Surg. 1993;165(1):9–14.
Flum DR, Dellinger EP, Cheadle A, Chan L, Koepsell T. Intraoperative cholangiography and risk of common bile duct injury during cholecystectomy. JAMA. 2003;289(13):1639–44.
Giger U, Ouaissi M, Schmitz SFH, Krähenbühl S, Krähenbühl L. Bile duct injury and use of cholangiography during laparoscopic cholecystectomy. Br J Surg. 2011;98(3):391–6.
Strasberg SM, Eagon CJ, Drebin JA. The “hidden cystic duct” syndrome and the infundibular technique of laparoscopic cholecystectomy–the danger of the false infundibulum. J Am Coll Surg. 2000;191(6):661–7.
Shang P, Liu B, Li X, Miao J, Lv R, Guo W. A practical new strategy to prevent bile duct injury during laparoscopic cholecystectomy. A single-center experience with 5539 cases. Acta Cir Bras. 2020;35(6):e202000607.
Strasberg SM, Brunt LM. Rationale and use of the critical view of safety in laparoscopic cholecystectomy. J Am Coll Surg. 2010;211(1):132–8.
Avgerinos C, Kelgiorgi D, Touloumis Z, Baltatzi L, Dervenis C. One thousand laparoscopic cholecystectomies in a single surgical unit using the “critical view of safety” technique. J Gastrointest Surg. 2009;13(3):498–503.
Tsalis K, Antoniou N, Koukouritaki Z, Patridas D, Christoforidis E, Lazaridis C. Open-access technique and “critical view of safety” as the safest way to perform laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech. 2015;25(2):119–24.
Dili A, Bertrand C. Laparoscopic ultrasonography as an alternative to intraoperative cholangiography during laparoscopic cholecystectomy. World J Gastroenterol. 2017;23(29):5438–50.
Dili A, Bertrand C. Laparoscopic ultrasonography as a good alternative to intraoperative cholangiography (IOC) during laparoscopic cholecystectomy: results of prospective study. World J Gastroenterol. 2017;23(29):5438–50.
Aziz O, Ashrafian H, Jones C, Harling L, Kumar S, Garas G, et al. Laparoscopic ultrasonography versus intra-operative cholangiogram for the detection of common bile duct stones during laparoscopic cholecystectomy: a meta-analysis of diagnostic accuracy. Int J Surg. 2014;12(7):712–9.
Matsumura M, Kawaguchi Y, Kobayashi Y, Kobayashi K, Ishizawa T, Akamatsu N, et al. Indocyanine green administration a day before surgery may increase bile duct detectability on fluorescence cholangiography during laparoscopic cholecystectomy. J Hepatobiliary Pancreat Sci. 2021;28(2):202–10.
Ishizawa T, Bandai Y, Ijichi M, Kaneko J, Hasegawa K, Kokudo N. Fluorescent cholangiography illuminating the biliary tree during laparoscopic cholecystectomy. Br J Surg. 2010;97(9):1369–77.
Osayi SN, Wendling MR, Drosdeck JM, Chaudhry UI, Perry KA, Noria SF, et al. Near-infrared fluorescent cholangiography facilitates identification of biliary anatomy during laparoscopic cholecystectomy. Surg Endosc. 2015;29(2):368–75.
Ishizawa T, Tamura S, Masuda K, Aoki T, Hasegawa K, Imamura H, et al. Intraoperative fluorescent cholangiography using indocyanine green: a biliary road map for safe surgery. J Am Coll Surg. 2009;208(1):e1-4.
Dip F, LoMenzo E, Sarotto L, Phillips E, Todeschini H, Nahmod M, et al. Randomized trial of near-infrared incisionless fluorescent cholangiography. Ann Surg. 2019;270(6):992–9.
Boogerd LSF, Handgraaf HJM, Huurman VAL, Lam H-D, Mieog JSD, van der Made WJ, et al. The best approach for laparoscopic fluorescence cholangiography: overview of the literature and optimization of dose and dosing time. Surg Innov. 2017;24(4):386–96.
Chen Q, Zhou R, Weng J, Lai Y, Liu H, Kuang J, et al. Extrahepatic biliary tract visualization using near-infrared fluorescence imaging with indocyanine green: optimization of dose and dosing time. Surg Endosc. 2021;35(10):5573–82.
Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–91.
Zarrinpar A, Dutson EP, Mobley C, Busuttil RW, Lewis CE, Tillou A, et al. Intraoperative laparoscopic near-infrared fluorescence cholangiography to facilitate anatomical identification: when to give indocyanine green and how much. Surg Innov. 2016;23(4):360–5.
Tsutsui N, Yoshida M, Nakagawa H, Ito E, Iwase R, Suzuki N, et al. Optimal timing of preoperative indocyanine green administration for fluorescent cholangiography during laparoscopic cholecystectomy using the PINPOINT® endoscopic fluorescence imaging system. Asian J Endosc Surg. 2018;11(3):199–205.
Dip F, Nguyen D, Montorfano L, Szretter Noste ME, Lo Menzo E, Simpfendorfer C, et al. Accuracy of near infrared-guided surgery in morbidly obese subjects undergoing laparoscopic cholecystectomy. Obes Surg. 2016;26(3):525–30.
Boni L, David G, Mangano A, Dionigi G, Rausei S, Spampatti S, et al. Clinical applications of indocyanine green (ICG) enhanced fluorescence in laparoscopic surgery. Surg Endosc. 2015;29(7):2046–55.
Verbeek FPR, Schaafsma BE, Tummers QRJG, van der Vorst JR, van der Made WJ, Baeten CIM, et al. Optimization of near-infrared fluorescence cholangiography for open and laparoscopic surgery. Surg Endosc. 2014;28(4):1076–82.
Acknowledgements
We thank all participants for joining this trial, and all anesthesiologists of Guangzhou First People's Hospital for their cooperation during surgery.
Funding
This trial was supported in part by the Guangzhou Key Clinical Specialty Project (2019SZDZK01), Guangzhou Clinical Characteristic Technology Project (2019TS44) and Science and Technology Projects in Guangzhou (202102010027, 202201010623).
Author information
Authors and Affiliations
Contributions
Study concept and design: YH, QC, JK, SZ, WG; Recruiting and supporting participants: YH, JW, YL, ZW, HL, DH, FL, GZ; Data analysis and interpretation: YH, QC, WG; Drafting the manuscript: YH; Critical revision of the manuscript for intellectual content: YH, QC, TC, WG;
Corresponding author
Ethics declarations
Conflict of interest
We have no conflicts of interest to declare.
Ethical approval
This study was approved by the Ethics Committee of Guangzhou First People’s Hospital (No. K-2022–033-01). This study was registered in the Chinese Clinical Trial Registry (ChiCTR2200057933). Written informed consent was obtained from all participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
595_2022_2563_MOESM1_ESM.tiff
Supplementary file1 Introduction of the OptoMedic 2100 Series HD fluorescence endoscopic system. (A) Photographs of the fluorescence endoscopic system. (B) Images in different imaging modalities. Refer to the following link for detailed information: http://www.optomedic.com/product/detail/1 (TIFF 3295 KB)

595_2022_2563_MOESM3_ESM.tiff
Supplementary file3 Example of the measurement of fluorescent intensity in the common bile duct (CBD) and liver. (A) The indocyanine green (ICG) image and the greyscale image in the CBD and liver. (B) Selection of a region of interest (ROI) in the CBD (ROI_1). (C) Selection of an ROI in the liver (ROI_2). (D) Measuring the area, the mean grey value, and the minimum and maximum gray values of the ROIs. The BLR was calculated with mean grey value as the ratio of ROI_1 to ROI_2 (TIFF 2516 KB)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, Y., Chen, Q., Kuang, J. et al. Real-time fluorescent cholangiography with indocyanine green in laparoscopic cholecystectomy: a randomized controlled trial to establish the optimal indocyanine green dose within 30 min preoperatively. Surg Today 53, 223–231 (2023). https://doi.org/10.1007/s00595-022-02563-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-022-02563-y