Abstract
Background
There have been no studies to systematically evaluate the two display (3D vs. 2D) systems regarding both laparoscopic and thoracoscopic surgeries in clinical settings; thus, we conducted one to evaluate the safety and efficacy of different visualization systems (two-dimensional and three-dimensional) during endoscopic surgery (laparoscopy or thoracoscopy) in clinical settings.
Methods
A comprehensive search of online databases was performed. Perioperative outcomes were synthesized. Cumulative meta-analysis was performed to evaluate the temporal trend of pooled outcomes. Specific subgroups (laparoscopy vs. thoracoscopy, prospective vs. retrospective study, malignant vs. benign diseases) were examined. Meta-regression was conducted to explore the source of heterogeneity.
Results
Twenty-three articles were considered in this analysis, of which 7 were thoracoscopic and 16 were laparoscopic surgeries. A total of 2930 patients were recorded, of which 1367 underwent 3D video-assisted surgery and 1563 underwent 2D display. Overall, significantly shorter operating time (SMD −0.69; p = <0.001), less blood loss (SMD −0.26; p = 0.028) and shorter hospital stays (SMD −0.16; p = 0.016) were found in the 3D display group. Meanwhile, the perioperative morbidity (OR 0.92; p = 0.487), retrieved lymph nodes (SMD 0.09; p = 0.081), drainage duration (SMD −0.15; p = 0.105) and drainage volume (SMD 0.00; p = 0.994) were similar between the two groups. Comparison of the overall outcomes in each subset showed consistency in all groups.
Conclusions
This up-to-date meta-analysis reveals that the 3D display system is superior to the 2D system in clinical settings with significantly shorter operating time, less blood loss and shorter hospital stay. These findings suggest that, in laparoscopic or thoracoscopic surgeries, 3D endoscopic system is preferable when condition permits. Future efforts should be made on decreasing the side effects of 3D display and increasing its cost-effectiveness.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In an attempt to explore more minimally invasive operation methods, the video-assisted endoscopic approach, including both laparoscopic and thoracoscopic surgeries, has been rapidly developed and widely adopted. Using conventional two-dimensional (2D) displays, endoscopic surgeons rely on indirect visual cues in the form of relative and expected motion, shadows, textures and relative color differences to extract indirect three-dimensional (3D) information, which leads to the loss of depth perception and consequently a more cognitive workload for surgeons [1]. The employment of stereoscopic 3D displays is the apparent solution to overcome these technical obstacles and improve endoscopic skills compared with conventional 2D systems, for both experienced and non-experienced endoscopic surgeons [2].
Even though 3D displays have not yet replaced 2D displays due, in part, to the conflicting evidence in the literature addressing the benefit of 3D displays and their perceptual challenges, [3, 4] several systematic reviews have evaluated 2D and 3D displays applied with laparoscopy in simulated settings. These reviews observed shorter operative times, higher early continence rates, lower error rates and a shorter learning curve for novices, but still drawing obscure conclusions [5, 6]. Furthermore, the majority of these comparative studies were conducted based only on laparoscopic surgery and in simulated settings; hitherto, no study has systematically evaluated the two display systems regarding both laparoscopic and thoracoscopic surgeries with clinical cases.
With the aim of comprehensively evaluating 3D versus 2D display systems in clinical settings, we conducted a systematic review and meta-analysis in both video-assisted laparoscopic and thoracoscopic surgeries, including the assessment of operating time, blood loss volume, drainage duration, drainage volume, 30-day mortality, morbidity, conversion rate to open, duration of hospitalization and the number of lymph nodes retrieved.
Methods
Literature search and selection
A systematic and comprehensive literature search of online databases PubMed, Web of Science, MEDLINE and Cochrane Library was performed to identify observational studies and RCTs performed before August 2017 that simultaneously examined the comparative studies of 2D and 3D display video-assisted surgery (laparoscopic or thoracoscopic surgery).
Several search terms and related variants were used, including 2D, 3D, 2-dimentional, 3-dimentional, stereoscopic, monoscopic, laparoscopy, thoracoscopy and surgery. The references of identified papers, previous published systematic reviews and meta-analyses were inspected to identify studies not included by the initial search.
We evaluated all searched results according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement [7]. The selection of original studies was based on the process of viewing titles, abstracts and full papers. Inclusion criteria were as follows: (1) studies including surgery operated with laparoscopy or thoracoscopy; (2) comparative studies examining 3D versus 2D displays; (3) RCTs or observational (cohort and case–control) studies if they were comparative in nature; (4) studies that reported at least one outcome of interest. Non-comparative studies, review articles, abstracts, case reports, editorials, expert opinions, commentary articles, studies with simulated setting, robotic-assisted surgery and letters were excluded.
Data extraction and quality assessment
Data were extracted independently by two investigators (H. R. Liang and C. Z. Liu), and conflicts were adjudicated by a third investigator (W. H. Liang). For the selected studies, information on all available variables was extracted and entered into a Microsoft Excel database. The following outcomes were used to compare the two operative techniques: (a) intraoperative parameters: operating time, blood loss volume, conversion rate, lymph nodes retrieved; (b) postoperative data: drainage duration, drainage volume, duration of hospitalization, 30-day mortality and morbidity; (c) population and study design details. We performed subgroup analyses which were stratified by surgical procedures (laparoscopy vs. thoracoscopy), study designs (prospective study vs. retrospective study) and disease type (benign vs. malignant diseases). The Joanna Briggs Institute Prevalence Critical Appraisal Tool was used to assess the quality of included studies [8]. Any disagreement was resolved via discussion among the authors.
Statistical analysis
Odds ratio (OR) with 95% confidence intervals (95% CIs) was calculated for categorical outcomes (morbidity). Standardized mean difference (SMD) with 95% CI was calculated for continuous outcomes (operating time, blood loss volume, drainage duration, drainage volume, duration of hospitalization and the number of lymph nodes retrieved). We used Cochran’s Chi-square test and I2 to examine the heterogeneity among effect estimates. Statistical heterogeneity among studies was defined as I2 statistic greater than 50% [9]. Fixed effects model was preferred to random effects model when there was no statistically significant heterogeneity and vice versa when there was significant heterogeneity [10].
When necessary, mean and standard deviations were estimated from the available median and confidence interval or range [11]. Study bias was detected using the methods of funnel plot, and Begg’s and Egger’s test [12]. A sensitivity analysis was performed by excluding the studies with the lowest-quality score. Cumulative meta-analyses were performed according to published time order to assess the stabilization of the effect sizes. Meta-regression was conducted to explore the source of heterogeneity. Statistical significance was taken as two-sided p < 0.05. The analysis was performed with STATA 12.0 software (Stata Corporation, College Station, TX, USA).
Results
Study selection and quality assessment
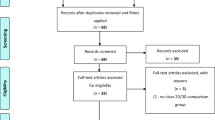
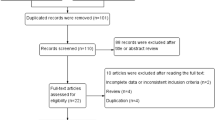
A total of 161 records were screened after exclusion of duplicates up to July 31, 2017. Finally, 23 full-text articles reporting safety and efficacy of 2D versus 3D video-assisted surgery met the inclusion criteria and were considered in this analysis. Of the included articles 7 were of thoracoscopic surgery [13,14,15,16,17,18,19] and 16 were of laparoscopic surgery [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35] (Fig. 1). There were three groups of outcomes according to different diseases by Ji et al. [30], and the three groups were regarded independently in following analyses.
The contextual details and the results of the quality assessment of each study are summarized in Table 1. The studies were conducted in 7 different countries with the period ranging from 2006 to 2017. Three RCTs, 4 prospective cohorts and 16 retrospective observational studies were included. A total of 2930 patients were recorded, of which 1367 underwent 3D video-assisted surgery and 1563 underwent conventional 2D video-assisted surgery. All studies achieved 7–10 stars in quality assessment on a scale of 0–10. Table 2 shows the demographics of included studies.
Intraoperative parameters
All studies mentioned operating time, which was significantly shorter in the 3D display group (SMD −0.69; 95% CI −0.91 to −0.47; p = <0.001), with significant heterogeneity (I2 = 86.3%, p = < 0.001) (Fig. 2a). Less blood loss was shown in 3D as well (SMD −0.26; 95% CI −0.49 to −0.03; p = 0.028) (I2 = 87.2%, p = < 0.001) (Fig. 2b). In addition, using the fixed model (I2 = 46.2%, p = 0.040), the number of retrieved lymph nodes seemed to increase when applying 3D visualization compared with the 2D system (SMD 0.09; 95% CI −0.01 to 0.18; p = 0.081), though statistically insignificant (Fig. 2c).
Postoperative parameters
Meta-analysis demonstrated that the perioperative morbidity was similar between patients who underwent 2D and 3D display procedures (OR 0.92; 95% CI 0.73–1.16; p = 0.487), with no heterogeneity (I2 = 0%, p = 0.652) (Fig. 3a). Hospital stay was significantly shorter in the 3D group (SMD −0.16; 95% CI −0.29 to −0.03; p = 0.016), but the heterogeneity was significant (I2 = 55.8%, p = 0.002) (Fig. 3b).
Drainage duration and drainage volume
Nine studies provided information on drainage duration, and six studies gave drainage volume data. Random model was used in analysis. There were no significant statistics in both drainage duration (SMD −0.15; 95% CI −0.32 to 0.03; p = 0.105) and drainage volume (SMD 0.00; 95% CI −0.42 to 0.42; p = 0.994) regarding 3D versus 2D display groups. And the heterogeneities were significant in the two outcomes (I2 = 65.6, p = 0.003; I2 = 90.7, p = < 0.001) (Fig. 4a, b).
30-day mortality and conversion rate to open
The 30-day mortality was defined as death within 30 days of the operation or any time after operation if the patient did not leave the hospital alive in all included studies. Conversion was defined as the use of a rib-spreading thoracotomy or laparotomy at any point after initiation of thoracoscopic or laparoscopic surgery. Thirteen studies provided information on 30-day mortality, and 17 provided the intraoperative conversion rate; however, the majority of studies reported no cases of death or conversion. In total, 1/879 death occurred in the 3D display group, and 3/1013 deaths occurred in the 2D surgery group, while 14/1050 versus 18/1194 cases of conversion occurred in 3D and 2D display groups, respectively.
Subgroup analysis and cumulative meta-analysis
Outcomes were separated into several subsets according to surgical procedures (laparoscopy vs. thoracoscopy), study designs (prospective vs. retrospective study) and disease type (benign vs. malignant diseases). Comparing with the overall outcomes in each subset, all groups showed consistency (Online Supplementary 1). Cumulative meta-analysis of each set of outcomes demonstrated that as more studies were added, the CI narrowed and the effect size became stable (Online Supplementary 2).
Assessment of side effects
Only one study [14] indicated though no surgeons reported nausea or headaches, several circulating nurses who were too close to the screen complained of dizziness and eye fatigue during the beginning of the operation. This discomfort generally disappeared after 5 min. Another three studies [20, 25, 28] stated that none of the surgeons complained of visual strains, dizziness or headache during or after the 3D procedure. Others did not note.
Publication bias, sensitivity analysis and meta-regression
Visual inspection of funnel plots suggested a symmetric distribution of main studies (Online Supplementary 3). Begg’s and Egger’s test confirmed there was no significant publication bias (Online Supplementary 4). A sensitivity analysis was performed by excluding the studies with the lowest-quality score. This did not influence the results. We have also conducted meta-regression with the covariates of published year, study types, endoscopy types, disease types, quality score and different regions. All these covariates were statistically insignificant in regard to their effect on heterogeneity.
Discussion
Endoscopy with 3D imaging has existed as an alternative to conventional video-assisted surgery for over 20 years. The camera system in modern 3D endoscopes consists of two adjacent cameras, which simulates the stereopsis obtained from the fusion of the slightly different views from the binocular disparity of the two human eyes, known as stereoscopy. To the best of our knowledge, this is the first systematic review and meta-analysis to evaluate the safety and efficacy of different endoscopic (laparoscopy or thoracoscopy) visualization systems (2D and 3D) in clinical settings.
As described above, although not all the studies were RCTs or prospective studies, the majority of the included studies were of moderate to high quality. Twenty-three comparative studies with 2930 patients underwent 2D or 3D display video-assisted laparoscopic or thoracoscopic surgery. Our analysis reflects the latest surgical results. Overall, significantly shorter operating time (SMD −0.69; p = <0.001), less blood loss (SMD −0.26; p = 0.028) and shorter hospital stay (SMD −0.16; p = 0.016) were found in the 3D display group. Meanwhile, the perioperative morbidity (OR 0.92; p = 0.487), the number of retrieved lymph nodes (SMD 0.09; p = 0.081), drainage duration (SMD −0.15; p = 0.105) and drainage volume (SMD 0.00; p = 0.994) were similar between the two groups. In total, 1/879 death and 14/1050 conversions occurred in the 3D display group, while 3/1013 deaths and 18/1194 conversions occurred in the 2D surgery group. Compared with the former systematic reviews [6, 36], our meta-analysis based on clinical settings provided more concrete, updated and comprehensive results, which indicated that 3D visualization performed superior than 2D display system during laparoscopic or thoracoscopic surgery.
Compared with the 2D endoscopic system, 3D endoscopy can provide three-dimensional vision that allows simpler presentation of anatomical structures, and better sense of depth to facilitate precise operation and shorten the operation time. The high-definition 3D vision also allows surgeons to quickly improve surgical skills and shorten the learning curve in simulated settings [37]. There is a significant improvement in the performance time of each training task by novice surgeons or specialists when working under 3D vision compared to 2D vision. Furthermore, the 3D system-simulated training program seemed to improve task efficiency, reduce the number of errors and accelerate basic thoracoscopic and laparoscopic skill acquisition, both for more experienced and new surgeons [5]. The current study could not assess the effects of 3D visualization on future performance, but we could hypothesize that participants may further benefit from learning endoscopic skills under the conditions of 3D visualization.
In our pooled analysis, hospital stay was shorter in the 3D group, while morbidity was similar between the two groups. As the matter of routine, hospitalization time prolonged with the increase in postoperative complications, but our study seems not to support the statement. We regard that it is type and severity of complications not total incidence rate that count. Though postoperative morbidity was similar between two groups, the spectrum of complications might be different. With 3D endoscopic display, blood loss was significantly reduced compared with conventional display; we have the reason to believe that 3D endoscopy tends to be associated with more moderate postoperative complication. Lu et al. [21] reported 3D laparoscopic surgery seems associated with increased overall complications than 2D (2D: 16.1%; 3D: 18.3%); on the contrary, major complications appeared more in 2D group (2D: 2.7%; 3D: 1.8%). Other studies also support the point of view [20, 25]. In addition, discharge time is a variate of great subjective of physicians; thus, 3D endoscopic surgery might offer more confidence to physicians in decreasing the hospital duration. For these reasons, it is no wired that 3D group has similar complication rate with 2D, but shorter hospital duration.
Though none of the surgeons complained of visual strains, dizziness or headache during or after the 3D procedure of enrolled studies in our analysis, 3D vision during endoscopy has previously been linked to side effects such as dizziness, headaches, eyestrain, disorientation and physical discomfort [38]. A systematic review reported 18 trials measuring whether participants felt any side effects when using 3D vision technology. In the results half of the trials reported side effects. The most commonly investigated were discomfort, dizziness, eye strain, nausea and fatigue [36]. These side effects might decrease when using new and more advanced 3D systems with improved image quality, compared to the first generation of 3D systems which offered the surgeons degraded viewing conditions because of poor image resolution [39]. Furthermore, the first generation of 3D systems used heavy and uncomfortable eyewear, while the newest 3D glasses are light and comfortable to wear. The newly developed glasses-free three-dimensional endoscopic system may be a potential solution to overcome these side effects [40].
Aside from the conventional three-dimensional display system, 2 other 3D displays have emerged in recent years. 3D robotic-assisted surgery has been proved a feasible and safe alternative to conventional endoscopic surgery, with less 30-day mortality and morbidity in both laparoscopic or thoracoscopic surgeries [41, 42]. Furthermore, robotic-assisted surgery has the advantage of advanced features such as three-dimensional visualization, increased degree of motion and rotational freedom, and small-wristed instruments that facilitate complex movements in a rigid cavity [42]. Another new technology, glasses-free 3D endoscopic system, was developed recently and applied in video-assisted thoracoscopic surgery [40], which effectively reduced the operator’s sense of discomfort and poor lighting; meanwhile, it effectively improved the operator’s sense of space and reduced the time required for space conversions. Although the currently used 3D system is more expensive than the standard 2D system, the expense of operating the 3D robotic system or the glasses-free 3D display is even higher; thus, the conventional 3D endoscopic system may represent the optimum selection for many centers.
In all, the current study showed that 3D display endoscopic surgery was associated with significantly shorter operating time, less blood loss and shorter hospital stay than 2D display, which probably contributed to better sense of space and depth. Therefore, we believed that 3D endoscopic surgery should be considered a preferable option when economic condition permits, especially for surgeries with complex anatomical steps or with difficult procedures.
We acknowledge several limitations to our study. First, not all of the included studies were prospective randomized comparisons, which increase the risk of potential selection and reporting bias. Second, heterogeneity among studies still existed. We have conducted meta-regression of published year, study types, endoscopy types, disease types, quality score and different regions. All these covariates were statistically insignificant in regard to their effect on heterogeneity. The different levels of surgeons’ experience, the shorter learning curve for the 3D display group and the differing baseline characteristics of the two groups might explain the heterogeneity. In addition, 15 out of 23 articles were conducted in China, which can be another bias for the article. Last, the data we used are based on the published literature rather than the primary data as we were unable to obtain unpublished data.
Conclusions
The meta-analysis of current evidence reveals that the 3D display system is superior to the 2D system in clinical settings with significantly shorter operating time, less blood loss and shorter hospital stay. These findings suggest that, in laparoscopic or thoracoscopic surgeries, 3D endoscopic system is preferable when condition permits. Future efforts should be made on decreasing the side effects of 3D display and increasing its cost-effectiveness.
References
Hanna GB, Shimi SM, Cuschieri A (1998) Randomised study of influence of two-dimensional versus three-dimensional imaging on performance of laparoscopic cholecystectomy. Lancet (London, England) 351(9098):248–251
Sakata S, Watson MO, Grove PM, Stevenson AR (2016) The conflicting evidence of three-dimensional displays in laparoscopy: a review of systems old and new. Ann Surg 263(2):234–239
Chan AC, Chung SC, Yim AP, Lau JY, Ng EK, Li AK (1997) Comparison of two-dimensional vs three-dimensional camera systems in laparoscopic surgery. Surg Endosc 11(5):438–440
Taffinder N, Smith SG, Huber J, Russell RC, Darzi A (1999) The effect of a second-generation 3D endoscope on the laparoscopic precision of novices and experienced surgeons. Surg Endosc 13(11):1087–1092
Ozsoy M, Kallidonis P, Kyriazis I, Panagopoulos V, Vasilas M, Sakellaropoulos GC, Liatsikos E (2015) Novice surgeons: do they benefit from 3D laparoscopy? Lasers Med Sci 30(4):1325–1333
Fergo C, Burcharth J, Pommergaard HC, Kildebro N, Rosenberg J (2017) Three-dimensional laparoscopy vs 2-dimensional laparoscopy with high-definition technology for abdominal surgery: a systematic review. Am J Surg 213(1):159–170
Knobloch K, Yoon U, Vogt PM (2011) Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Cranio-Maxillo-Fac Surg Off Publ Eur Assoc Cranio-Maxillo-Fac Surg 39(2):91–92
Munn Z, Moola S, Riitano D, Lisy K (2014) The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag 3(3):123–128
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5:13
Seagroatt V, Stratton I (1998) Bias in meta-analysis detected by a simple, graphical test. Test had 10% false positive rate. BMJ 316(7129):470 (author reply 470-471)
Jiao P, Wu QJ, Sun YG, Ma C, Tian WX, Yu HB, Tong HF (2017) Comparative study of three-dimensional versus two-dimensional video-assisted thoracoscopic two-port lobectomy. Thorac Cancer 8(1):3–7
Yang CL, Wang W, Mo LL, Zhang L, Peng GL, Yu ZW, Liu YY, He JX (2016) Short-term outcome of three-dimensional versus two-dimensional video-assisted thoracic surgery for benign pulmonary diseases. Ann Thorac Surg 101(4):1297–1302
Dong S, Yang XN, Zhong WZ, Nie Q, Liao RQ, Lin JT, Wu YL (2016) Comparison of three-dimensional and two-dimensional visualization in video-assisted thoracoscopic lobectomy. Thorac Cancer 7(5):530–534
Yang C, Mo L, Ma Y, Peng G, Ren Y, Wang W, Liu Y, He J (2015) A comparative analysis of lung cancer patients treated with lobectomy via three-dimensional video-assisted thoracoscopic surgery versus two-dimensional resection. J Thorac Dis 7(10):1798–1805
Li Z, Li JP, Qin X, Xu BB, Han YD, Liu SD, Zhu WZ, Peng MZ, Lin Q (2015) Three-dimensional vs two-dimensional video assisted thoracoscopic esophagectomy for patients with esophageal cancer. World J Gastroenterol 21(37):10675–10682
Hou Y, Guo W, Yang Z, Zhao J (2015) Comparative study of 3D thoracoscopic esophagectomy versus 2D thoracoscopic esophagectomy for esophageal carcinoma. Zhonghua wei chang wai ke za zhi = Chin J Gastrointest Surg 18(9):889–892
Bagan P, De Dominicis F, Hernigou J, Dakhil B, Zaimi R, Pricopi C, Le Pimpec Barthes F, Berna P (2015) Complete thoracoscopic lobectomy for cancer: comparative study of three-dimensional high-definition with two-dimensional high-definition video systems dagger. Interact CardioVasc Thorac Surg 20(6):820–823
Padin EM, Santos RS, Fernandez SG, Jimenez AB, Fernandez SE, Dacosta EC, Duran AR, Artime Rial M, Dominguez Sanchez I (2017) Impact of three-dimensional laparoscopy in a bariatric surgery program: influence in the learning curve. Obes Surg 27:2552–2556
Lu J, Zheng CH, Zheng HL, Li P, Xie JW, Wang JB, Lin JX, Chen QY, Cao LL, Lin M et al (2017) Randomized, controlled trial comparing clinical outcomes of 3D and 2D laparoscopic surgery for gastric cancer: an interim report. Surg Endosc 31(7):2939–2945
Kanaji S, Suzuki S, Harada H, Nishi M, Yamamoto M, Matsuda T, Oshikiri T, Nakamura T, Fujino Y, Tominaga M et al (2017) Comparison of two- and three-dimensional display for performance of laparoscopic total gastrectomy for gastric cancer. Langenbeck’s Arch Surg 402(3):493–500
Ji F, Fang X, Fei B (2017) Comparative study of 3D and 2D laparoscopic surgery for gastrointestinal tumors. Zhonghua wei chang wai ke za zhi = Chin J Gastrointest Surg 20(5):509–513
Zeng N, Fang C, Yang J, Xiang N, Zhu W, Liu J, Chen Q, Liang H, Huang W (2016) Application of three-dimensional laparoscopic cholecystectomy for complicated gallstone disease. Nan fang yi ke da xue xue bao = J South Med Univ 36(1):145–147
Velayutham V, Fuks D, Nomi T, Kawaguchi Y, Gayet B (2016) 3D visualization reduces operating time when compared to high-definition 2D in laparoscopic liver resection: a case-matched study. Surg Endosc 30(1):147–153
Tao K, Liu X, Deng M, Shi W, Gao J (2016) Three-dimensional against 2-dimensional laparoscopic colectomy for right-sided colon cancer. Surg Laparosc Endosc Percutaneous Tech 26(4):324–327
Tang FJ, Qi L, Jiang HC, Tong SY, Li Y (2016) Comparison of the clinical effectiveness of 3D and 2D imaging systems for laparoscopic radical cystectomy with pelvic lymph node dissection. J Int Med Res 44(3):613–619
Ruan Y, Wang XH, Wang K, Zhao YY, Xia SJ, Xu DL (2016) Clinical evaluation and technical features of three-dimensional laparoscopic partial nephrectomy with selective segmental artery clamping. World J Urol 34(5):679–685
Komatsuda A, Matsumoto K, Miyajima A, Kaneko G, Mizuno R, Kikuchi E, Oya M (2016) Technical improvement using a three-dimensional video system for laparoscopic partial nephrectomy. Asian Pac J Cancer Prev APJCP 17(5):2475–2478
Ji G, Qi S, Ji F, Tao Y, Ma C, Fang X (2016) Comparative study of three-dimensional and two-dimensional laparoscopic-assisted D2 radical gastrectomy in short-term efficacy. Zhonghua wei chang wai ke za zhi = Chin J Gastrointest Surg 19(5):545–548
Abou-Haidar H, Al-Qaoud T, Jednak R, Brzezinski A, El-Sherbiny M, Capolicchio JP (2016) Laparoscopic pyeloplasty: Initial experience with 3D vision laparoscopy and articulating shears. J Pediatr Urol 12(6):426.e421–426.e425
Bove P, Iacovelli V, Celestino F, De Carlo F, Vespasiani G, Finazzi Agro E (2015) 3D vs 2D laparoscopic radical prostatectomy in organ-confined prostate cancer: comparison of operative data and pentafecta rates: a single cohort study. BMC Urol 15:12
Zou Z, Huang Z, Li Q, Chen F, Zhao D, Wang M (2014) A comparative study of three-dimensional versus two-dimensional laparoscopic subtotal thyroidectomy via a breast approach. Nan fang yi ke da xue xue bao = J South Med Univ 34(8):1233–1234
Xu W, Li H, Ji Z, Zhang X, Zhang Y, Xiao H, Liu G (2014) Comparison of three dimensional and two dimentional laparoscopic pyeloplasty for ureteropelvic junction obstruction. Zhonghua wai ke za zhi = Chin J Surg 52(10):771–774
Aykan S, Singhal P, Nguyen DP, Yigit A, Tuken M, Yakut E, Colakerol A, Sulejman S, Semercioz A (2014) Perioperative, pathologic, and early continence outcomes comparing three-dimensional and two-dimensional display systems for laparoscopic radical prostatectomy–a retrospective, single-surgeon study. J Endourol 28(5):539–543
Sorensen SM, Savran MM, Konge L, Bjerrum F (2016) Three-dimensional versus two-dimensional vision in laparoscopy: a systematic review. Surg Endosc 30(1):11–23
Kyriazis I, Ozsoy M, Kallidonis P, Vasilas M, Panagopoulos V, Liatsikos E (2015) Integrating three-dimensional vision in laparoscopy: the learning curve of an expert. J Endourol 29(6):657–660
Wilhelm D, Reiser S, Kohn N, Witte M, Leiner U, Muhlbach L, Ruschin D, Reiner W, Feussner H (2014) Comparative evaluation of HD 2D/3D laparoscopic monitors and benchmarking to a theoretically ideal 3D pseudodisplay: even well-experienced laparoscopists perform better with 3D. Surg Endosc 28(8):2387–2397
Sorensen SMD, Konge L, Bjerrum F (2017) 3D vision accelerates laparoscopic proficiency and skills are transferable to 2D conditions: a randomized trial. Am J Surg 214(1):63–68
Shao W, Yin W, Wang W, Zhang X, Peng G, Chen X, Mo L, He J (2016) Glasses-free three-dimensional endoscopic bronchoplasty, arterioplasty, and angioplasty of the superior vena cava for the radical treatment of right middle upper lung cancer. J Thorac Dis 8(3):608–611
Liang H, Liang W, Zhao L, Chen D, Zhang J, Zhang Y, Tang S, He J (2017) Robotic versus video-assisted lobectomy/segmentectomy for lung cancer: a meta-analysis. Ann Surg. https://doi.org/10.1097/SLA.0000000000002346
Maeso S, Reza M, Mayol JA, Blasco JA, Guerra M, Andradas E, Plana MN (2010) Efficacy of the Da Vinci surgical system in abdominal surgery compared with that of laparoscopy: a systematic review and meta-analysis. Ann Surg 252(2):254–262
Acknowledgements
All authors were involved in the conception and design of the study. H-R L and Z-C L contributed to the data acquisition. H-R L, W-H L, Y-H C and Z-C L contributed to the analysis and writing of the manuscript. All authors critically reviewed and approved the final manuscript. We thank Lindsey Hamblin for assistance with the language revision.
Funding
This work was supported by the following funding: Science and Technology Planning Project of Guangdong Province, China (Grant Numbers: 2007B031515017; 2008A030201024); Science and Technology Planning Project of Guangzhou, China (Grant Numbers: 2007Z1-E0111; 2007Z3-E0261); and Guangzhou Health and Medical Collaborative Innovative Major Special Projects (Grant No. 201400000001-2)
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liang, H., Liang, W., Lei, Z. et al. Three-Dimensional Versus Two-Dimensional Video-Assisted Endoscopic Surgery: A Meta-analysis of Clinical Data. World J Surg 42, 3658–3668 (2018). https://doi.org/10.1007/s00268-018-4681-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-018-4681-z