Abstract
Introduction
Modern perioperative care strategies aim to optimise perioperative care by reducing the body’s stress response to surgery. A major facet of optimising an abdominal surgery analgesia programme is using a multimodal opioid sparing approach. Local anaesthetics have shown promise and there has been considerable research into the most effective route for their administration. This review aims to determine if there is a difference in analgesic efficacy between intraperitoneal local anaesthetic (IPLA) and intravenous local anaesthetic (IVLA).
Materials and Methodology
In concordance with the PRISMA statement, a literature search was conducted to identify randomised control trials that compared IVLA with IPLA in abdominal surgery. The primary outcomes of interest were opioid analgesia requirements and pain score assessed by visual analogue score. Data were extracted and entered into pre-designed electronic spreadsheets.
Results
This review has identified six papers that compared intravenous lignocaine to intraperitoneal lignocaine. This review showed significantly lower morphine consumption at 4 and 24 h in the intraperitoneal group. There was no significant difference in pain scores.
Conclusion
From the analysis of these studies, intraperitoneal local anaesthetic had an analgesic benefit over intravenous lignocaine with regard to decreased opioid consumption for abdominal surgery. Further research investigating IVL combined with intraperitoneal local anaesthetic is warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Modern perioperative care strategies aim to optimise perioperative care by reducing the body’s stress response to surgery [1, 2]. Multimodal analgesia regimens are vital components of all such strategies as inadequate analgesia following major abdominal surgery results in poorer outcomes, poor quality of life, and longer length of stay (LOS) [3]. Historically, opiates have been at the heart of analgesia strategies in abdominal surgery.
Although opioids have excellent analgesic properties, they have a number of harmful side effects including post-operative nausea and vomiting (PONV), post-operative ileus and other complications [4]. These opioid-related side effects can lead to prolonged LOS [5]. Opioid abuse in the USA is at record levels, and there has been an increase in the number of patients developing opioid addiction after using them in the perioperative setting [6]. It is estimated that the cost of the opioid crisis on the American economy annually exceeds $78.5 billion [6]. Thus, viable alternatives to opioids with similar analgesic efficacy need to be developed.
A major facet of optimising an abdominal surgery analgesia programme is using a multimodal opioid sparing approach [7]. This has led to the experimental use of local anaesthetics, ketamine and gabapentinoids [8]. Local anaesthetics have shown promise, and there has been considerable research into the most effective route for their administration.
Two routes for local anaesthetics that have demonstrated promise are intravenous (IVLA) and intraperitoneal (IPLA) administration. IPLA infusion has been shown to be effective at reducing pain and improving early recovery after major abdominal surgery [9,10,11]. This is thought to be due, at least in part, to the creation of a transient chemical blockade of vagal afferents [10]. However, after a bolus of IPLA is given, local anaesthetic levels can be detected in the systemic system within 2–4 min [12, 13]. With such rapid absorption of the IPLA, it raises the question as to whether the analgesic benefit is local or systemic, or is there a combined or synergistic effect?
Studies comparing IPLA to IVLA have been limited to lignocaine due to the short half-life in the intravascular space [8]. There have been systematic reviews investigating the role of IPLA and IVLA as individual means of analgesia. However, no review has been performed directly comparing the two routes of administration. This review aims to determine if there is a difference in analgesic efficacy between IPLA and IVLA.
Methods
Search strategy
Two independent authors (WM and WX) performed the literature search using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement as a guide [14]. A comprehensive search of three electronic databases (PubMed, EMBASE, Cochrane Data base and Medline [ex ovid-medline)] was conducted, from inception to October 2017. The search terms were: ‘laparotomy or abdominal surgery or laparoscopic or abdomen or surgery’, and ‘IV or intravenous or systemic’, and ‘Intraperitoneal or Intra-peritoneal or peritoneum’, and ‘Local anaesthetic or local anaesthetic or lignocaine or lidocaine’. Limitations were kept to human subjects. All transcripts which were not in English were translated. Reference lists were manually examined to find any further studies. A hand search process was performed in journals, congress proceedings, and reference lists of included and excluded trials.
Study selection
All randomised clinical trials (RCT) were included in this review if they compared intravenous lignocaine to intraperitoneal lignocaine and they measured their effects on pain scores and opioid consumption. The inclusion criteria included adult patients (> 16 years) that underwent any form of abdominal surgery that had two separate groups that compared intravenous lignocaine to intraperitoneal lignocaine. Exclusion criteria were non-abdominal surgeries, studies that did not contain the primary outcomes, studies that were not available in full text (conference abstracts) and those that were not RCTs. Studies were examined by two independent authors to ensure they met the inclusion and exclusion criteria. Any disputes around the inclusion or exclusion criteria were discussed with the senior author, and the senior authors’ decision was final.
Data extraction
The data were extracted and entered into pre-designed electronic spread sheets. The primary outcomes were opioid consumption and pain scores. The secondary outcomes included length of hospital stay, serum lignocaine levels, inflammatory markers, functional gastrointestinal recovery, post-operative nausea or vomiting, post-operative complications and lignocaine toxicity. All opioid consumption was extracted and then converted to morphine equivalent doses (MED). Non-steroidal analgesia was not included in meta-analysis. Only studies that assessed pain scores using the visual analogue scale ((VAS), 0–100 mm or 0–10 cm) were analysed. Secondary outcomes were extracted where available.
Assessment of risk of bias
Each included study was appraised to assess the risk of bias as outlined in Sect. 8.5 of the Cochrane Handbook for Systematic Reviews of Interventions [15]. All studies were rated as at ‘low risk’, ‘unclear risk’ or ‘high risk’ of bias for each criterion.
Statistical analysis
Data were analysed using Review Manager version 5.3 [16]. For continuous variables, meta-analysis was conducted using the inverse variance method with a random effects model and outcomes recorded as the difference in means. Statistical significance was set at the 95% confidence interval (CI). Heterogeneity of the studies was assessed using I2.
Estimating missing data
If continuous data were reported as median and range, estimates of mean and standard deviation were calculated using a standardised validated tool [17]. Where standard error of mean was reported, these were converted to standard deviation. Data provided in the form of graphs were extracted using Plot Digitizer version 2.6.8 [18, 19].
Funding
This research was funded by The University of Auckland.
Results
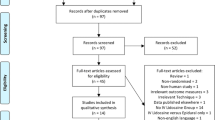
The literature search identified 338 potential articles for inclusion in this review (Fig. 1). Four studies from the search met the inclusion and exclusion criteria, and further two studies were identified through manual searches of the reference lists. A total of 509 patients were identified in the six studies with 363 included in the analysis after placebo groups were removed. There were four different procedures examined in the studies. Two studies looked at laparoscopic cholecystectomy, two studies looked at abdominal hysterectomy, one study looked at caesarean section and one looked at laparoscopic appendicectomy [20,21,22,23,24,25].
Opioid consumption
All opioid analgesia used within each study was converted into morphine equivalent dose using equianalgesic dose ratios for opioids. The time points reported in each study varied. At 4 h only three of the studies reported opioid consumption (Fig. 2); however, all 6 studies did report analgesia consumption at 24 h (Fig. 3). IPLA was found to be more effective than IVLA. However, there was significant heterogeneity (SMD 2.39 (0.7, 4.07); P = 0.005; I2 = 97%) (Fig. 3).
Pain scores
Five studies reported pain scores at 4 and 24 h (Figs. 4, 5). Four studies reported pain scores at 8 h (Fig. 6), and four studies reported pain scores at 12 h (Fig. 7). The only time point that showed a significant difference between the two groups was at 24 h (SMD 0.22 (0, 0.45); P = 0.05, I2 = 59%) (Fig. 5).
Secondary outcomes
There was no significant difference in length of hospital stay in four studies, and it was not reported in two studies. Systemic lignocaine levels were reported in one study, and it showed lower levels in the intraperitoneal group. Inflammatory makers (C-reactive protein and leucocyte count) were reported in one study, and it showed no significant difference. Nausea and vomiting were not shown to be significant in any other included studies. Time of return of gastrointestinal function was not reported in one study, not significant in four studies and lower in the IV group for one study (Table 1).
Toxicity
The shortest infusions were for the length of the procedure and longest were run for 1 h post-operatively. There were no prolonged post-operative infusions. There were no reported episodes of lignocaine toxicity in any of the six studies.
Risk of bias
When the Cochrane risks of bias tool scores were converted to Agency for Health and Research Quality (AHRQ) standards, four studies were classified as good quality. One study was classified as fair quality, and one study was classified as poor quality (Fig. 8).
Discussion
This review has identified six papers that compared intravenous lignocaine to intraperitoneal lignocaine in the context of abdominal surgery. The results of this review showed significantly lower morphine consumption at 4 and 24 h in the intraperitoneal group. There was no significant difference in pain scores. No toxicity was seen across the six studies. Intraperitoneal lignocaine is worthy of consideration in multimodal analgesia regimes for analgesia following abdominal surgery.
The analgesic benefit of intravenous local anaesthetic (IVLA) was described as early as 1958. However, patients were at high risk of adverse side effects with the initially trialled doses [26]. Over the last decade, IVLA has had a revival with better understanding of safe dosing. It is proposed that through its opioid-sparring effect, anti-inflammatory properties and decreasing of inhibitory sympathetic tone, IVLA improves pain and bowel function leading to a shorter hospital stay after abdominal surgery [8]. In order to achieve an analgesic benefit in the perioperative setting with intravenous lignocaine, a bolus is required prior to an infusion. The described dose for the bolus is 1–2 mg kg−1 and for the infusion it is 0.5–3 mg kg−1 h−2 [8].
The use of IVLA has been reviewed in all abdominal surgeries. A Cochrane review found moderate evidence that there was improvement in pain scores especially when compared to placebo in the early post-operative phase, but there was limited evidence that it had any benefit for other clinical outcomes [27]. A recent systematic review that looked at intravenous lignocaine specifically in colorectal surgery found similar findings. However, many of the included studies did not give post-operative infusions. The context-sensitive half-time after a 3-day infusion of lidocaine is ~ 20–40 min may explain the early analgesic benefit; however, the benefit seems to extend further [8]. Koppert suggests this may be due to the additional antihyperalgesic effect of systemic lignocaine [28]. Thus far, IVLA use has been restricted to lignocaine. There is hesitation to use longer-acting local anaesthetics intravenously due to the safety aspects of their longer half-life.
Longer-acting local anaesthetics such as bupivacaine and ropivacaine have been utilised with good effect intraperitoneally [9, 29, 30]. A recent systematic review of seven studies supported the use of IPLA with reduced pain scores compared to placebo. Kahokehr demonstrated these effects with a study that compared IPLA with epidural versus epidural + placebo in open colorectal procedures and found that the IPLA group had lower pain scores and opioid consumption and early improvement in recovery [10]. A similar study that used IPLA in laparoscopic colorectal cases also showed lower pain scores, opioid consumption and better recovery scores in the IPLA group. An overview of systematic reviews examining IPLA in abdominal surgery found that the analgesic benefit was stronger for gastric and gynaecological procedures than for laparoscopic cholecystectomies [31]. This is significant as one-third of trials included in this review were laparoscopic cholecystectomies. This could have been a contributing factor as to why there was a difference in opioid consumption but not in pain scores.
The mechanism of action of IPLA is not fully understood. It has been postulated that IPLA acts by creating a transient chemical block of vagal afferents at the site of surgical dissection. This blocks the gut–brain axis that transmits both painful and nociceptive stimuli and hence decreases the neuroendocrine response to the surgical injury [32]. This has been demonstrated in animal studies where a vagotomy has been shown to decrease this response [33, 34].
The analgesic benefit of IPLA may not only be restricted to its local effect but may be mediated through systemic effects. A systematic review of nine studies that looked at systemic levels of lignocaine after an intraperitoneal bolus revealed systemic levels can be detected at as early as 5 min and had a T-max that ranged from 15 to 40 min [12]. Levels from the boluses ranged from 1.1 to 4.32 µg ml−1 which would sit in the described therapeutic window of 2.5–3.5 µg ml−1 and below the level for potential side effects (5 µg ml−1) [8, 12].
There are several limitations to this review. Firstly, although five of the papers were ranked as low risk of bias, there were still only 363 patients who were included in this review. This puts the data at risk of overestimating or missing a treatment effect. Secondly, patients either received no post-operative infusion or an infusion for only 1–2 h while in the recovery area after surgery. The limited infusion might influence the results as the half-life of lignocaine is 20–40 min. Thirdly, there was significant heterogeneity between the studies that may be at least partially explained by failure to standardise pain regimens. Another limitation of this paper is the analytical methods used. The software used was Review Manager which does not allow for t-statistic (Satterthwaite correction). The heterogeneity experienced would arise from different protocols as well as different types of operations (some in upper and some in lower abdomen). The heterogeneity can be explained clinically with inherent differences in the studies, so further statistical corrections were not sought. Finally, within the six included studies there were four different procedures and none of those procedures were colorectal or urological.
In conclusion, there are analgesic benefits when local anaesthetics are utilised in a multimodal analgesic regime. From the analysis of these studies, intraperitoneal local anaesthetic had an analgesic benefit over intravenous lignocaine with regard to decreased opioid consumption for abdominal surgery. More studies are required to look at its use in urological, colorectal and upper gastrointestinal procedures. Further studies investigating and comparing the utility of more prolonged infusions in a broader range of procedures would seem to be indicated.
References
Kahokehr A, Sammour T, Zargar-Shoshtari K et al (2009) Implementation of ERAS and how to overcome the barriers. Int J Surg 7:16–19
Kehlet H, Wilmore DW (2005) Fast-track surgery. Br J Surg 92:3–4
Boulind CE, Ewings P, Bulley SH et al (2013) Feasibility study of analgesia via epidural versus continuous wound infusion after laparoscopic colorectal resection. Br J Surg 100:395–402
Choi YY, Park JS, Park SY et al (2015) Can intravenous patient-controlled analgesia be omitted in patients undergoing laparoscopic surgery for colorectal cancer? Ann Surg Treat Res 88:86–91
Artinyan A, Nunoo-Mensah JW, Balasubramaniam S et al (2008) Prolonged postoperative ileus-definition, risk factors, and predictors after surgery. World J Surg 32:1495–1500. https://doi.org/10.1007/s00268-008-9491-2
Hah JM, Bateman BT, Ratliff J et al (2017) Chronic opioid use after surgery: implications for perioperative management in the face of the opioid epidemic. Anesth Analg 125:1733–1740
Zargar-Shoshtari K, Hill AG (2008) Optimization of perioperative care for colonic surgery: a review of the evidence. ANZ J Surg 78:13–23
Eipe N, Gupta S, Penning J (2016) Intravenous lidocaine for acute pain: an evidence-based clinical update. BJA Education 16:292–298
Kahokehr A (2013) Intraperitoneal local anesthetic for postoperative pain. Saudi J Anaesth 7:5
Kahokehr A, Sammour T, Shoshtari KZ et al (2011) Intraperitoneal local anesthetic improves recovery after colon resection: a double-blinded randomized controlled trial. Ann Surg 254:28–38
Kahokehr A, Sammour T, Soop M et al (2010) Intraperitoneal use of local anesthetic in laparoscopic cholecystectomy: systematic review and metaanalysis of randomized controlled trials. J Hepato-Biliary-Pancreat Sci 17:637–656
Kahokehr A, Sammour T, Vather R et al (2010) Systemic levels of local anaesthetic after intra-peritoneal application—a systematic review. Anaesth Intensive Care 38:623–638
Fuhrer Y, Charpentier C, Boulanger G et al (1996) Analgesia after laparoscopic cholecystectomy by intraperitoneal administration of bupivacaine. Ann Fr Anesth Reanim 15:128–134
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535
Higgins JP, Altman DG, Gotzsche PC et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928
(RevMan) RM. Review Manager (RevMan) (2004) In: Collaboration TC editor. The Nordic Cochrane Centre, Copenhagen
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Meth 5:13
Jelicic Kadic A, Vucic K, Dosenovic S et al (2016) Extracting data from figures with software was faster, with higher interrater reliability than manual extraction. J Clin Epidemiol 74:119–123
Digitizer P (2015) Plot Digitizer In: sourceforge.net editor, sourceforge.net
Kim TH, Kang H, Hong JH et al (2011) Intraperitoneal and intravenous lidocaine for effective pain relief after laparoscopic appendectomy: a prospective, randomized, double-blind, placebo-controlled study. Surg Endosc Other Interv Tech 25:3183–3190
Ram D, Sistla SC, Karthikeyan VS et al (2014) Comparison of intravenous and intraperitoneal lignocaine for pain relief following laparoscopic cholecystectomy: A double-blind, randomized, clinical trial. Surg Endosc Other Interv Tech 28:1291–1297
Yang SY, Kang H, Choi GJ et al (2014) Efficacy of intraperitoneal and intravenous lidocaine on pain relief after laparoscopic cholecystectomy. J Int Med Res 42:307–319
Samimi S, Taheri A, Tanha F (2015) Comparison between intraperitoneal and intravenous lidocaine for postoperative analgesia after elective abdominal hysterectomy, a double-blind placebo controlled study. J Fam Reprod Health 9:193–198
Perniola A, Fant F, Magnuson A et al (2014) Postoperative pain after abdominal hysterectomy: A randomized, double-blind, controlled trial comparing continuous infusion vs patient-controlled intraperitoneal injection of local anaesthetic. Br J Anaes 112:328–336
Murad AFM, Abosrie M, Alazeem E, Mostafa A (2016) Efficacy of intraperitoneal versus intravenous lidocaine for postcesarean pain relief. Evidence based womens health journal 6:144–148
De Clive-Lowe SG, Desmond J, North J (1958) Intravenous lignocaine anaesthesia. Anaesthesia 13(138–14):6
Kranke P, Jokinen J, Pace NL et al (2015) Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD009642.pub2
Koppert W, Weigand M, Neumann F et al (2004) Perioperative intravenous lidocaine has preventive effects on postoperative pain and morphine consumption after major abdominal surgery. Anesth Analg 98:1050–1055
Kahokehr A, Sammour T, Zargar K et al (2011) Intraperitoneal local anaesthetic in colon resection—A double-blinded randomised controlled trial. Colorectal Dis 13:59
Kahokehr A, Sammour T, Soop M et al (2010) Intraperitoneal use of local anesthetic in laparoscopic cholecystectomy: systematic review and metaanalysis of randomized controlled trials. J Hepatobiliary Pancreat Sci 17(5):637–656
Hamill JK, Rahiri JL, Hill AG (2017) Analgesic effect of intraperitoneal local anesthetic in surgery: an overview of systematic reviews. J Surg Res 212:167–177
Kahokehr A, Sammour T, Srinivasa S et al (2011) Metabolic response to abdominal surgery: the 2-wound model. Surgery 149:301–304
Traub RJ, Sengupta JN, Gebhart GF (1996) Differential c-fos expression in the nucleus of the solitary tract and spinal cord following noxious gastric distention in the rat. Neuroscience 74:873–884
Berthoud HR, Neuhuber WL (2000) Functional and chemical anatomy of the afferent vagal system. Auton Neurosci Basic Clin 85:1–17
Author information
Authors and Affiliations
Contributions
WSM designed the study, performed initial screening and review of all articles included, and composed the manuscript. WX assisted in the study design, performed screening and review of all articles included, and assisted in the preparation of the final manuscript. AB assisted in the study design and performed statistical analysis. BS assisted in the study design and in the preparation of the final manuscript. DS is the co-supervisor and assisted in the preparation of the final manuscript. AGH is the senior author and principal investigator, and provided supervision to the co-authors.
Corresponding author
Rights and permissions
About this article
Cite this article
MacFater, W.S., Xia, W., Barazanchi, A. et al. Intravenous Local Anaesthetic Compared with Intraperitoneal Local Anaesthetic in Abdominal Surgery: A Systematic Review. World J Surg 42, 3112–3119 (2018). https://doi.org/10.1007/s00268-018-4623-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-018-4623-9