Abstract
Background
Enhanced recovery after surgery (ERAS) colorectal guideline implementation has occurred primarily in standalone institutions worldwide. We implemented the guideline in a single provincial healthcare system, and our study examined the effect of the guideline on patient outcomes [length of stay (LOS), complications, and 30-day post-discharge readmissions] across a healthcare system.
Methods
We compared pre- and post-guideline implementation in consecutive elective colorectal patients, ≥18 years, from six Alberta hospitals between February 2013 and December 2014. Participants were followed up to 30 days post discharge. We used summary statistics, to assess the LOS and complications, and multivariate regression methods to assess readmissions and to estimate cost impacts.
Results
A total of 1333 patients (350 pre- and 983 post-ERAS) were analysed. Of this number, 55 % were males. Median overall guideline compliance was 39 % in pre- and 60 % in post-ERAS patients. Median LOS was 6 days for pre-ERAS compared to 4.5 days in post-ERAS patients with the longest implementation (p value <0.0001). Adjusted risk ratio (RR) was 1.71, 95 % CI 1.09–2.68 for 30-day readmission, comparing pre- to post-ERAS patients. The proportion of patients who developed at least one complication was significantly reduced, from pre- to post-ERAS, difference in proportions = 11.7 %, 95 % CI 2.5–21.0, p value: 0.0139. The net cost savings attributable to guideline implementation ranged between $2806 and $5898 USD per patient.
Conclusion
The findings in our study have shown that ERAS colorectal guideline implementation within a healthcare system resulted in patient outcome improvements, similar to those obtained in smaller standalone implementations. There was a significant beneficial impact of ERAS on scarce health system resources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Enhanced recovery (ER) programs in surgery seek to improve patient outcomes by integrating an evidence-based guideline, an implementation program, and engaging health care practitioners to modify practice within a health system or site [1–5]. Audit processes support and enhance practice change at both the provider and system level. The enhanced recovery after surgery (ERAS®) Society colorectal guideline has been widely studied internationally [6–11]. The most current meta-analysis [11] showed an impact on patient outcomes including reduction in length of stay (LOS, average of 2.5 days), decrease in complications [10–13] and cost benefits to the system (mean savings of 1651€ ($2245 USD) per patient) [9, 14, 15]. However, national data on LOS in many Western countries show that LOS is still at 8 days or more for elective colonic resections, a suggestion that ERAS guideline implementation is slow. While some sites believe they use ERAS, only few have full control over the practice and cannot provide credible data on outcomes; moreover, only a few have an ERAS program and if they do, they may not know the guideline compliance rate. Although quality registries can provide annual reports to inform site-based practice, the use of a more active and continuous audit is rarely employed.
Internationally, ERAS implementation has largely occurred within a single site. There has been one large system-wide reported attempt to implement multiple guidelines for elective colorectal, orthopaedic, gynaecologic and urologic surgery, in the United Kingdom’s Enhanced Recovery Partnership Program (ERPP). A recent report from ERPP suggests that stringent delivery of a consistent care pathway leads to greater health benefit for the patient and a cost savings for the healthcare organisation [1]. ERPP focused on thorough patient preparation for surgery: use of minimally invasive surgery, optimal fluid and pain management and rapid reintroduction of oral nutrition and mobilisation. However, in this study there was little change in guideline compliance over the study period, yet a dose response effect was reported, with higher guideline compliance being weakly associated with increased reduction in LOS. Although there was funding for education, implementation and data collection a structured implementation program never existed and data collection was voluntary. The authors concluded that outcomes improved with higher compliance; hence, a stringent implementation system should guide practice. The ERAS® Society has developed an implementation program that was piloted in the Netherlands with good results [16], further enhanced with the addition of an interactive and continuous audit, by the local ERAS team, and this proved useful in single centres in Europe [9]. This implementation program is currently in use in several countries in six continents, mostly in single units rather than across a health care system.
In Alberta, Canada, we are in the process of employing the ERAS Society’s structured implementation program within a single health care system for colorectal surgery. ERAS Alberta is funded by the provincial health system which services over 4 million people in 59 acute care facilities. The initial phase consists of six participating sites that perform over 75 % of all colorectal surgery in the province. The implementation and integration of a multisite ERAS colorectal strategy is a unique opportunity to tailor the ERAS® Society’s Implementation Program (EIP) and customise provider uptake within the province.
This study aims to evaluate the initial impact of the ERAS® Society’s colorectal guideline in a systematic implementation program on patient outcomes and to assess guideline compliance across multiple sites within a single healthcare system. In addition, this preliminary work was undertaken to support the creation of a knowledge translation framework to inform spread and scale of ER programs.
Methods
The Alberta Health Services (AHS) ERAS® Implementation Program (EIP) began in February 2013 (Fig. 1) starting with two lead sites, Peter Lougheed Centre (PLC) and Grey Nuns Hospital (GNH). Subsequent to this, ERAS was further implemented at four more hospitals with high annual colorectal surgery volumes (range 150–346 cases/year): Royal Alexandra Hospital (RAH), Misericordia Community Hospital (MCH), University of Alberta Hospital (UAH) and Foothills Medical Centre (FMC).
The AHS EIP consisted of (i) forming an “implementation team” with a surgeon (local leader in practice), anaesthesiologist and nurse; (ii) collecting pre-ERAS (baseline) data for a minimum of 50 consecutive patients; (iii) entering pre-ERAS data into the ERAS® Interactive Audit System (EIAS); (iv) auditing pre-ERAS baseline data followed by tailored training from the ERAS Society to achieve international standards; (v) preparing for implementation where teams determined how they would change practice within their site (e.g. creating new order sets); (vi) prospectively recruiting consecutive patients and (vii) auditing perioperative practice biweekly by site, using EIAS to examine guideline compliance. The ERAS® Society colorectal guideline includes 22 care elements in the preoperative, intraoperative and postoperative areas and is linked to EIAS at each participating site (Table 1).
Outcomes
Data on patient outcomes, demographics, and other clinical elements were entered into EIAS. The main outcome measures were LOS (number of days between primary operation date and discharge date), complications (grouped by EIAS categories: urological, respiratory, infectious, cardiovascular, renal, hepatic, pancreatic, gastrointestinal, surgical, anaesthetic and psychiatric, Table 2) and readmissions (readmission within 30 days post discharge). Compliance for each of the 22 care elements in the pre-, intra- and postoperative phases of the perioperative period was also recorded.
Sample size estimate
With a standard deviation of 11 days, calculated from historic data from the two lead sites (PLC and GNH), a confidence level of 0.05, and a power of 80 %, an estimated sample size of 475 patients could detect an expected mean difference of 2 days between pre- and post-ERAS patients [10, 11]. Using the rule of at least ten observations per predictor variable in multiple regression models [17, 18], this sample size enabled use of regression models that included all potential confounding factors, for LOS, complications and readmissions as outcome variables.
Data analyses
At the time of data analysis, the AHS EIP was fully implemented for 15 months at the PLC and GNH, 5 months at the UAH and RAH, and 4 months at the FMC and MCH. Wilcoxon tests were used to compare pre- and post-ERAS age, BMI and overall ERAS compliance, while Chi-square tests, with post hoc Bonferroni corrections, were used for comparing multinomial variables. Given a lack of maturity and of outcome data at the newer sites, only PLC and GNH (lead sites with the longest ERAS implementation history) data were used to assess the effect of ERAS implementation on patient outcomes (LOS, complications, readmissions). Patients were grouped into five categories of 3-month time intervals and LOS was compared between pre- and post-ERAS according to these categories, using the Wilcoxon test. Patients were also divided into two groups according to complexity of the operation—group 1 (surgically more complex): abdominoperineal resection, anterior resection of rectum, total/subtotal colectomy, reversal of Hartmann’s procedure; and group 2 (surgically less complex): right hemicolectomy, left hemicolectomy, other large/small bowel resection, ileostomy reversal. A log-binomial regression model was used to compute the risk ratio (RR) for 30-day post-discharge readmission in post-ERAS compared to pre-ERAS patients, adjusted for potential confounding factors (surgical approach, ASA class, surgery type, gender, smoking status, alcohol consumption and diabetes status). Chi-square tests were used to compare the proportions of patients who developed at least one complication (by complications group) during primary hospital stay in post-ERAS compared to pre-ERAS patients. For exploratory purposes, post-ERAS patients’ guideline compliance was categorised into quintiles: compliance ≤43 % (compliance 1), compliance 44–52 % (compliance 2), compliance 53–61 % (compliance 3), compliance 62–71 % (compliance 4) and compliance ≥72 % (compliance 5). Summary statistics with post hoc Bonferroni corrections were used to assess possible dose–response dependence in LOS and complications. Summary statistics were also used to assess reoperations, and intensive care usage (ICU).
A cost impact analysis, using a hospital perspective, examined primary LOS (i.e. for surgery), 30-day post-discharge readmissions and readmission LOS, to determine potential differences in costs at the two lead sites. To calculate the net cost impact, we subtracted the ERAS intervention costs including labour/coordination and licensing fees.
Differences in LOS and in readmission likelihood were estimated as described above. Cost impacts were estimated by applying unit costs of an inpatient hospital stay to the differences between pre- and post-ERAS patients. Unit costs of an inpatient hospital stay were estimated from the Alberta hospital discharge abstract database using the case mix groups of colorectal surgery. The unit cost of an inpatient hospital stay was estimated to be $947 to $1790. Note that these costs exclude costs of physician services and pharmaceuticals. All costs are reported in US Dollars (USD)—in 2014, the purchasing power parity (PPP) for gross domestic income from the United States/Canada bilateral program was 85.0 US cents per Canadian dollar.
In a sensitivity analysis, differences in LOS were estimated by multivariate negative binomial regressions. Differences in the likelihood of readmission were estimated by a multivariate logistic regression. These analyses were adjusted for age, gender and BMI, stage of cancer, type of cancer, ASA physical status class, preoperative chemotherapy, surgical approach, blood loss, main procedure, comorbidities, hospital site (GNH vs. PLC), smoking and alcohol consumption.
Ethics approval was obtained from the Research Ethics Boards at the University of Calgary and University of Alberta, Canada.
Results
Demographic characteristics, diagnosis, surgical approach and ASA class for all six sites are described in Table 3. The median age was 62 and 64 years at pre- and post-ERAS, respectively. Participants were generally overweight, median BMI = 27.5. There was a balance in age, BMI, gender and ASA class between pre- and post-ERAS patients, with 99 % of them belonging to ASA class 1, 2 and 3. Primary adenocarcinoma and benign tumour were the main final diagnoses. Open surgeries decreased from pre- to post-ERAS, while laparoscopic surgeries increased. Breakdown by surgical complexity was as follows: group 1 (surgically more complex): n = 533; group 2 (surgically less complex): n = 800. There were 54 % of patients at pre-ERAS and 60 % at post-ERAS in group 1, while 46 % of patients at pre-ERAS and 40 % at post-ERAS were in group 2 (p = 0.2108).
ERAS compliance
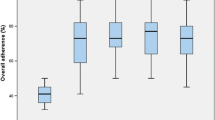
Median overall guideline compliance was 39 % in pre- compared to 60 % in post-ERAS patients. Preoperative elements had the highest compliance (median was 57 % in pre- and 83 % in post-ERAS patients), while postoperative elements had the lowest compliance (median was 19 % in pre- and 46 % in post-ERAS patients). Compliance with intraoperative care elements was similar between pre- and post-ERAS patients (median was 75 %), Fig. 2. Laparoscopic patients were more likely to have a higher overall guideline compliance (median overall ERAS compliance = 61.9 %), compared to open surgery patients (median overall ERAS compliance = 58.3 %) (Wilcoxon test, p value: <0.0001). Compliance increased rapidly during the first 3 months of implementation but stayed almost constant thereafter, Fig. 3.
Radar diagram comparing protocol compliance between pre- and post-ERAS patients. Energy intake 1 energy intake on day of surgery, postoperatively, energy intake 2 energy intake on postoperative day 1, mobilisation 1 mobilisation at all on day of surgery, mobilisation 2 mobilisation on postoperative day 1, mobilisation 3 mobilisation on postoperative day 2, mobilisation 4 mobilisation on postoperative day 3
LOS
There was a significant reduction in LOS from a pre-ERAS mean of 9.8 days (median 6 days) to a post-ERAS mean of 7.5 days (median 5 days). The LOS reduction followed a steady trend from 3 months of implementation onwards, Table 4, where Pre-ERAS is the comparator. LOS was reduced significantly from a pre-ERAS median of 6 days to a 15-month median of 4.5 days. Laparoscopic patients had a higher LOS reduction with a median LOS of 5 (mean = 7.5 days) and 4 (mean = 5.3) days pre- and post-ERAS, respectively, p value: <0.0001, compared to open surgical patients, median LOS = 8.5 (mean = 11.3 days) and 7 (mean = 10.4) days pre- and post-ERAS respectively, p value: 0.0723. For group 1 (surgically complex) procedures, there was a significant reduction in LOS from a pre-ERAS median LOS of 5 (mean = 9) days to a post-ERAS median LOS of 4 (mean = 6.9) days, p = 0.0020. For patients undergoing group 2 (surgically less complex) procedures, the pre-ERAS median LOS of 8 (mean = 10.8) days was reduced to a post-ERAS median LOS of 5.5 (mean = 8.3) days, p = 0.0002.
There were 9 (6.9 %) pre-ERAS compared to 23 (3.3 %) post-ERAS patients who spent at least one night in the ICU. The mean ± standard deviation LOS in ICU was 0.4 ± 1.8 days for pre-ERAS compared to 0.2 ± 1.8 days for post-ERAS patients and the change was not significant (Wilcoxon test, p value: 0.0514).
Complications
There was 56.9 % (95 % CI 48–65 %) of patients at pre-ERAS and 45.3 % (95 % CI 42–49 %) at post-ERAS who developed at least one complication and the difference in proportions of 11.7 %, 95 % CI 2.45 %–21.0 % was significant, Pearson Chi-square, p value = 0.0139, Table 5. For patients who had a group 1 (surgically more complex) procedure, 46.5 % (95 % CI 34.6–58.7 %) at pre-ERAS and 38.3 % (95 % CI 33.6–43.1 %) at post-ERAS developed at least one complication. The difference in proportions of 8.2 % (95 % CI −4.3–20.7 %) was not significant, p = 0.1912. For patients who underwent a group 2 (surgically less complex) procedure, 70 % (95 % CI 56.8–81.2 %) of pre-ERAS and 55.6 % (95 % CI 49.5–61.5 %) of post-ERAS patients developed at least one complication and the difference in proportions of 14.4 % (95 % CI 1.4–27.4 %) was significant, p = 0.0403.
For open surgery patients, 75.4 % (95 % CI 62.2–85.9 %) of pre- and 62.2 % (95 % CI 55.3–68.8 %) of post-ERAS patients developed at least one complication and a difference in proportions of 13.2 % (95 % CI 0.27–26.2 %) was only borderline significant 0.0632. For laparoscopic patients, 40.7 % (95 % CI 28.1–54.3 %) at pre-ERAS and 34.8 % (95 % CI 29.9–40 %) at post-ERAS developed at least one complication. The difference in proportions of 5.9 % (95 % CI −7.6–19.3 %) was not significant, p = 0.3839.
The most significant reduction was in respiratory complications, with a difference in proportions of 9.2, (95 % CI 2.9–15.5 %), followed by infectious complications, difference in proportion = 8.8, (95 % CI 1.9–15.7 %), Table 5. There was no significant difference in reoperations in pre-ERAS compared to post-ERAS patients (3.9 and 4.0 %, respectively; Chi-square test, p value: 0.9271).
A dose–response relationship was observed between increase in guideline compliance and reduction in LOS and in the proportion of patients who developed at least one complication. Compared to patients with compliance ≤43 % (compliance 1), patients with compliance 53–61 % (compliance 3), compliance 62–71 % (compliance 4) and compliance ≥72 % (compliance 5) were significantly less likely to develop any complication, Fig. 4.
Compared to patients with compliance ≤43 %, those with compliance ≥72 % were significantly more likely to have a shorter LOS. Patients with compliance ≥72 % were also significantly more likely to have a shorter LOS compared to patients with compliance 44–52 and 53–61 %, Fig. 4.
Readmissions
There was a total of twenty-two 30-day post-discharge readmissions (17.5 %) in pre-ERAS compared to 65 (9.6 %) in post-ERAS patients. There was a significant reduction in the risk of readmission, comparing pre- to post-ERAS patients (adjusted RR = 1.73, 95 % CI 1.09–2.73, p value = 0.0178). The 30-day post-discharge readmission risk was 1.73 times higher in pre- compared to post-ERAS patients. This association was significantly influenced by the surgical approach. Compared to open surgery patients, laparoscopic patients were less likely to be readmitted (RR = 0.62, 95 % CI 0.41–0.94, p value = 0.0241).
Costs
ERAS was associated with reducing the primary LOS at the two lead sites by 2.3 days equating to 1603 hospital days capacity. Readmissions were reduced by 7.9 % equating to 55 prevented readmissions and 660 hospital days. For those patients that were readmitted, ERAS was associated with reducing the LOS by 4.5 days equating to 293 hospital days. The total estimated gross cost savings were $2,420,276 to $4,575,496. The total cumulative intervention cost of ERAS during the analysis period was $464,518. The net cost savings of ERAS were therefore $1,955,758 to $4,110,977 or $2806 to $5898 per patient. Comparably, results of the sensitivity analysis showed the net cost savings per patient were $2668 to $5643.
Discussion
Systematic implementation of the ERAS colorectal guideline across a large healthcare system yielded benefits similar to those obtained in smaller single-site implementations. In the two sites with the longest ERAS implementation history, there was a significant improvement in patient outcomes (LOS, complications and readmissions) when comparing pre- to post-ERAS. Health benefits demonstrated in our study are similar to those already reported [10–13].
All patient outcomes (LOS, complications, readmissions) measured in this study were influenced by the surgical approach. Laparoscopic patients were more likely to have a shorter LOS, less likely to be readmitted and had fewer complications. For open surgery patients, there was no significant reduction in LOS between pre- and post-ERAS, an observation similar to published randomised controlled trials [19, 20]. However, our results also showed that, compared to laparoscopic patients, open surgery patients were more likely to have a lower overall ERAS compliance. This might have contributed to a lower impact on outcomes in this cohort of patients compared to laparoscopic patients. Although compliance to the ERAS guideline does not seem to differ between laparoscopic and open surgery patients in other studies [19, 21, 22], these studies reported that improvement in patient outcomes (reduction in LOS and complications) was dependent on the surgical approach. These findings are similar to those in our study. Laparoscopy, coupled with ERAS care, has been shown to significantly predict improved patient outcomes [19, 23, 24]. A dose–response association, similar to the one observed in our study, has been reported between patient outcomes improvement and ERAS guideline compliance by other researchers [1, 23, 25].
There are some important differences between the ERPP study [1] and ours. In our study, all consecutive elective colorectal patients were included in data collection and analysis. In the ERPP study, data collection was voluntary and it is not known if analysed data came from consecutive patients. We used a structured implementation program, including a regular biweekly team audit of data on compliance and outcomes, while this was not the case with the ERPP. In the ERPP, there was a reduction in LOS for both colonic and rectal resections by about 2 days, with LOS reduced to 8 and 10 days, respectively. There was, however, no clear data in the ERPP study [1] showing these figures. According to compliance and LOS data presented in the ERPP study, the best median LOS achieved was 6 days for patients with guideline compliance >90 %. In our approach, we found a continuous improvement over time with the final LOS for a combined colorectal population of a median of 4.5 days. This matches the level reported in trials [11] that studied solely laparoscopic surgery. In our study, open and laparoscopic surgeries were combined.
These significant improvements to patient outcomes have major implications on the health system in terms of health system efficiency and cost savings. A preliminary economic analysis indicated that after accounting for intervention costs, the reductions in LOS, complications and readmissions generated a net cost savings ranging between $1,955,758 to $4,110,977 or $2806 to $5898 per patient. Accordingly, the magnitude of the cost savings when ERAS is scaled within and between other types of surgeries is likely substantial. A fuller economic evaluation studying both the economic impact of ERAS on the colorectal experience as well as when it scales to other surgery types is forthcoming. These early results signal a significantly beneficial impact on scarce health system resources and warrant the attention of senior health system decision makers.
This “real world” study demonstrates the feasibility of implementing ERAS and impacting patient, system and economic outcomes within a large publically funded health care system. Rapid implementation and improvements in compliance with ERAS guidelines were achieved through both provincial leadership and local team ownership of the practice changes. Collaboration by teams between sites promoted joint problem-solving and sharing of learnings. Provincial leadership facilitated a systems approach and development of tools and resources to support implementation.
This study has a number of strengths. First, it was based on a large sample size of consecutive patients in a single health care system. Second, this study was based on a system-wide provincial initiative to change surgical care according to best evidence. The aim was to improve patient outcomes while reducing cost, which supports the provincial generalisability of the findings. Thirdly, a real-time interactive audit system for guideline implementation and data collection was used. Real-time data checks helped teams improve and maintain high compliance to the protocol as well as maintain high-quality data. Data were collected by well-trained nurse clinicians with in-depth knowledge of clinical aspects of colorectal surgery and outcomes measured in this study.
This study’s limitation is that eligible patients of colorectal surgeons who did not participate in the ERAS protocol were not analysed. However, this was only a very small population of patients. There is no reason to postulate that the benefits of ERAS implementation would have been different in these patients. Future work will include the analysis of these patients, using administrative data.
Conclusion
This study demonstrated that the impact of a system-wide ERAS implementation is no different from single-site implementation. Difficulties have been reported with compliance to some ERAS colorectal protocols [26–28] but the ERAS Alberta sites did not have any of these. Achieving compliance to the protocol elements occurred over time with a corresponding increase in benefits to patient outcomes. Total overall compliance in ERAS patients in the study was 60 %, indicating that there is room for improvement. Findings in this study identified lower compliance to postoperative care elements, thereby illustrating the greatest opportunity for practice change across the health care team, facilitated through audit and feedback. Future work includes identifying barriers/enablers to the implementation of ERAS in large healthcare systems to facilitate spread and scale of ER strategies.
References
Simpson JC, Moonesinghe SR, Grocott MPW et al (2015) Enhanced recovery from surgery in the UK: an audit of the enhanced recovery partnership programme 2009–2012. Br J Anaesth 115(4):560–568
Gustafsson UO, Scott MJ, Schwenk W et al (2013) Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. World J Surg 37:259–284. doi:10.1007/s00268-012-1772-0
Gustafsson UO, Scott MJ, Schwenk W et al (2012) Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr 31:783–800
Nygren J, Thacker J, Carli F et al (2013) Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. World J Surg 37:285–305. doi:10.1007/s00268-012-1787-6
Nygren J, Thacker J, Carli F et al (2012) Guidelines for perioperative care in elective rectal/pelvic surgery: enhanced recovery after surgery (ERAS®) Society recommendations. Clin Nutr 31:801–816
Walter CJ, Collin J, Dumville JC et al (2009) Enhanced recovery in colorectal resections: a systematic review and meta-analysis. Int J Colorectal Dis 11:344–353
Wind J, Polle SW, Jin PHPFK et al (2006) Systematic review of enhanced recovery programmes in colonic surgery. Br J Surg 93:800–809
Gouvas N, Tan E, Windsor A et al (2009) Fast-track vs standard care in colorectal surgery: a meta-analysis update. Int J Colorectal Dis 24:1119–1131
Adamina M, Kehlet H, Tomlinson GA et al (2011) Enhanced recovery pathways optimize health outcomes and resource utilization: a meta-analysis of randomized controlled trials in colorectal surgery. Surgery 149:830–840
Varadhan KK, Neal KR, Dejong CHC et al (2010) The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: a meta-analysis of randomized controlled trials. Clin Nutr 29:434–440
Greco M, Capretti G, Beretta L et al (2014) Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg 38:1531–1541. doi:10.1007/s00268-013-2416-8
Lv L, Y-f Shao, Y-b Zhou (2012) The enhanced recovery after surgery (ERAS) pathway for patients undergoing colorectal surgery: an update of meta-analysis of randomized controlled trials. Int J Colorectal Dis 27:1549–1554
Eskicioglu C, Forbes S, Aarts M-A et al (2009) Enhanced recovery after surgery (ERAS) programs for patients having colorectal surgery: a meta-analysis of randomized trials. J Gastrointest Surg 13:2321–2329
Roulin D, Donadini A, Gander S et al (2013) Cost-effectiveness of the implementation of an enhanced recovery protocol for colorectal surgery. Br J Surg 100:1108–1114
Lee L, Mata J, Ghitulescu GA et al (2014) Cost-effectiveness of enhanced recovery versus conventional perioperative management for colorectal surgery. Ann Surg 00:1–8
Gillissen F, Hoff C, Maessen JC et al (2013) Structured synchronous implementation of an enhanced recovery program in elective colonic surgery in 33 Hospitals in The Netherlands. World J Surg 37:1082–1093. doi:10.1007/s00268-013-1938-4
Bell GV (2008) Sample size. In: Statistical rules of thumb, vol 36, 2nd edn. John Wiley & Sons Inc., Hoboken, pp 1–14
Vittinghoff E, McCulloch CE (2007) Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol 165:710–718
Derksen S, Keselman HJ (1992) Backward, forward and stepwise automated subset selection algorithms: frequency of obtaining authentic and noise variables. Br J Math Stat Psychol 45:265–282
Vlug MS, Wind J, Hollmann MW et al (2011) Laparoscopy in combination with fast track multimodal management is the best perioperative strategy in patients undergoing colonic surgery: a randomized clinical trial (LAFA-study). Ann Surg 254:868–875
Delaney C, Zutshi M, Senagore A et al (2003) Prospective, randomized, controlled trial between a pathway of controlled rehabilitation with early ambulation and diet and traditional postoperative care after laparotomy and intestinal resection. Dis Colon Rectum 46:851–859
Gustafsson UO, Tiefenthal M, Thorell A et al (2012) Laparoscopic-assisted and open high anterior resection within an ERAS protocol. World J Surg 36:1154–1161. doi:10.1007/s00268-012-1519-y
Spanjersberg WR, Van Sambeeck JDP, Bremers A et al (2015) Systematic review and meta-analysis for laparoscopic versus open colon surgery with or without an ERAS programme. Surg Endosc 29(12):3443–3453
Group (2015) The impact of enhanced recovery protocol compliance on elective colorectal cancer resection: results from an international registry. Ann Surg 261:1153–1159
Van Bree S, Vlug M, Bemelman W et al (2011) Original research: faster recovery of gastrointestinal transit after laparoscopy and fast-track care in patients undergoing colonic surgery. Gastroenterology 141:872–880
Gustafsson UO, Hausel J, Thorell A et al (2011) Adherence to the enhanced recovery after surgery protocol and outcomes after colorectal cancer surgery. Arch Surg 146:571–577
Polle SW, Wind J, Fuhring JW et al (2007) Implementation of a fast-track perioperative care program: what are the difficulties? Dig Surg 24:441–449
Ahmed J, Khan S, Lim M et al (2012) Enhanced recovery after surgery protocols—compliance and variations in practice during routine colorectal surgery. Int J Colorectal Dis 14:1045–1051
Grant support
The ERAS project was funded by the Partnership for Research and Innovation in the Health System (PRIHS) research grant from Alberta Innovates: Health Solutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Ljungqvist is the current Chairman of the ERAS Society. He founded, serves on the Board and owns stock in Encare AB that runs the ERAS Society Interactive Audit System.
Additional information
Ljungqvist and Gramlich request shared senior authorship.
Rights and permissions
About this article
Cite this article
Nelson, G., Kiyang, L.N., Crumley, E.T. et al. Implementation of Enhanced Recovery After Surgery (ERAS) Across a Provincial Healthcare System: The ERAS Alberta Colorectal Surgery Experience. World J Surg 40, 1092–1103 (2016). https://doi.org/10.1007/s00268-016-3472-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-016-3472-7