Abstract
Background
The aim of this study was to determine the feasibility of image-guided marker-clip placement in axillary lymph nodes (ALNs) for breast cancer upon initial presentation and to assess the reliability of this method with sentinel lymph node biopsy (SLNB) for axillary restaging after neoadjuvant chemotherapy (NAC).
Methods
Between June 2015 and August 2016, a marker clip was placed at a clinically positive ALN under ultrasonography (US) guidance before initiation of NAC in 20 patients. Preoperative localization of marker-clipped LNs was performed, and the localized LNs were removed by SLNB. We compared the postoperative results of the marker-clipped LNs, SLNs and ALNs.
Results
Image-guided marker-clip placements and localization of marker-clipped LNs were performed successfully in 20 patients. A total of 24 marker clips were inserted, and 23 marker-clipped LNs were successfully retrieved during surgery (identification rate, 23/24, 95.8%). In the 11 patients with pathologically confirmed metastatic marker-clipped LNs, four became negative after NAC, and seven maintained metastatic residues on the marker-clipped LNs. Three of the seven patients had metastatic residues on the ALNs, and two of the three patients also had negative SLNs. Marker-clipped nodes accurately predicted the axillary nodal status in these two patients compared with SLNs alone.
Conclusion
Image-guided marker-clip placement on positive ALNs before NAC and removal with SLNB is technically feasible. This technique can improve the accuracy of the residual disease evaluation on the axilla, especially in patients with negative SLNB results, and can identify candidates for limited axillary surgery after NAC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Neoadjuvant chemotherapy (NAC) is used in patients with locally advanced breast cancer, especially large inoperable breast cancers. The advantages of this approach include downsizing of the primary tumor, which increases the success rate of breast-conserving therapy. The National Surgical Adjuvant Breast and Bowel Project (NSABP) Protocol B-18 demonstrated a negative conversion rate of nodal disease after NAC of up to 40% [1]. The extent of persistent axillary nodal disease after NAC is an important prognostic marker for locoregional recurrence and survival [2]. Nonetheless, there is no consensus on the most reliable method for restaging the axilla after NAC to confirm conversion to negative lymph node (LN) status.
Although sentinel LN biopsy (SLNB) reliably identifies LN metastases in women with clinically node-negative disease, this technique yields inconsistent identification rates and false-negative rates (FNRs) when performed in women with clinically node-positive disease who undergo NAC [3]. Because of the high false-negative rates (FNRs) of SLNBs reported in previous studies [4, 5], other modalities including axillary ultrasonography (US) with fine-needle aspiration (FNA), magnetic resonance imaging (MRI) and radioactive iodine seeds have been introduced to evaluate axillary LN status after NAC [6, 7]. The three largest studies of SLN surgery after NAC—American College of Surgeons Oncology Group (ACOSOG) Z1071, SN FNAC and SENTINA [8,9,10]—reported FNRs ranging from 8 to 14%. A recently published subgroup analysis of the ACOSOG Z1071 reported the lowest FNR of 6.8% after clip placement at positive LNs before NAC [11]. Likewise, the National Comprehensive Cancer Network (NCCN) guideline recommends marking axillary LNs with tattoos or clips before NAC and then removing them at the time of definitive surgery to reduce FNR [12]. Few recent studies have been published regarding targeted axillary dissection (TAD) after NAC.
Therefore, we aimed to determine the feasibility of image-guided marker-clip placement in positive ALNs upon initial presentation and to assess the reliability of this procedure for axillary restaging after NAC.
Methods
Patients
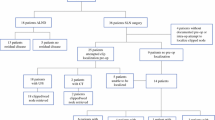
From June 2015 to August 2016, 312 patients were consecutively diagnosed with breast cancer, and 29 of these patients underwent NAC followed by surgery at Kangbuk Samsung Hospital. Patients were eligible if they had suspicious axillary LNs (the cortex was either focally or diffusely thickened (>3 mm thick), and the fatty hilum was deformed or absent) by US or PET-CT. Of the 21 patients who underwent LN-clip placement, one patient was excluded due to disease progression after three cycles of chemotherapy. Thus, 20 patients were included in this study. This single-institution prospective study was approved by the Institutional Review Board of Kangbuk Samsung Hospital (Approval No. 2015-03-043). Informed consent was obtained from all patients before initiation of the study.
US-guided FNA or core needle biopsies were performed on LNs with suspected metastasis before initiation of NAC. NAC was followed by breast-conserving surgery or mastectomy.
US-guided clip insertion
We prepared surgical clips with a disposable clip applier (LigaClip MCA MSM20, Ethicon Endo-Surgery, Somerville, NJ, USA; Premium Surgiclip M-9.75, Covidien, Mansfield, MA, USA) before initiation of the first NAC cycle. An 18-gauge (G) coaxial guiding needle (TSK Stericut; TSK Laboratory, Tochi-gi, Japan) was inserted into the cortex of the largest metastatic or suspicious LN, and the inner stylet was removed. A surgical clip was passed through the inserted introducer, and the inner stylet was reinserted to deploy the clip inside the LN (Fig. 1) [13]. One of four experienced breast-dedicated radiologists (4–22 years of experience) inserted and confirmed the placements of the clips. One day after the procedure and one day before surgery, unilateral digital mammography (Lorad Selenia; Hologic Inc., Danbury, CT, USA) of the mediolateral oblique (MLO) view was performed to objectively confirm the location of the inserted clip. In cases of non-visualization by mammography, we rechecked the fluoroscopic guide-captured image to verify the location of the inserted clip.
Wire localization of marker-clipped nodes
We performed wire localization 30 min to 1 h before surgery with a 21-G 7.5-cm hooked wire (Accura™ BLN; Argon, Athens, TX, USA) to retrieve the inserted clips from axillary LNs using cone beam computed tomography (CBCT, AlluraXper20/20; Philips Healthcare, Best, The Netherlands) by an experienced breast radiologist (Fig. 2).
a A marker clip was targeted with a localization needle using the shortest distance from the skin measured on the scout CBCT (long arrow: marker clip, short arrow: localization needle). b After targeting the clip, another angle of view was obtained to confirm the location of the needle tip (long arrow: marker clip, short arrow: localization needle)
On the angio-intervention table, in a supine position with both arms up, the first fluoroscopic-guided image was taken to confirm the location of the inserted clip and to select the appropriate field of view (FOV; 30–40 cm). Next, CBCT was performed for the selected region of interest with a single breath-hold. Acquired images were transferred to a workstation. We measured the distance from the clip to the skin. Under fluoroscopic guidance, the needle tip was placed at the marker-clipped LN and then hook-wire localization was performed. We repeatedly acquired CT images to confirm the location of the marker clip and needle tip (Fig. 3). The interval between clipping and surgery was 21 weeks for three patients with the TCHP chemotherapy regimen and 27 weeks for 17 patients with the AC+T(H) chemotherapy regimen.
Sentinel lymph node and marker-clipped lymph node surgery
A 99 mTc-labeled phytate colloid (Techne pyrophosphate kit inj., New Korea Industrial Co., Ltd, Seoul, Korea) was injected into the subdermal plexus of the breast 30 min before surgery (0.5 mCi). An indigo carmine dye (CARMINE INJ. 0.8%, United Pharm. Inc., Seoul, Korea) was injected into the subareolar plexus of the breast 5 min before surgery. SLNs were detected by the uptake of the radiolabeled colloid, the blue dye or both. The SLN surgery was considered successful if the surgeon identified nodes with radioactivity that was at least tenfold greater than the background count or a node that was blue. Intraoperative palpation and inspection of the specimen by a surgeon or specimen radiography by a radiologist confirmed that the excised LNs contained the clip (Fig. 4). After excision of the marker-clipped LNs, conventional SLNB proceeded. Axillary LN dissection (ALND) proceeded if more than two LNs (including marker-clip LNs and SLNs) were found to be metastatic during the intraoperative frozen biopsy [14].
Pathologic assessment
The nodal specimens were retrieved from the surgeon and delivered to a pathologist to identify marker-clipped LNs and to perform intraoperative evaluations. Marker-clipped nodes were grossly identified and serially sectioned at 2-mm intervals. The SLNs were processed in a similar manner. The serially sectioned LNs were embedded in an optimal cutting temperature compound and frozen. The LNs were subsequently sectioned at a 5 μm thickness with the microtome portion of the cryostat, and the sections were placed on glass slides and stained with hematoxylin and eosin. The pathologist evaluated the frozen sections and reported the results intraoperatively. Metastatic foci, including micrometastases, were considered node-positive, and isolated tumor cells were considered node-negative, according to the AJCC tumor-node-metastasis (TNM) staging system (7th edition) [15].
Results
Patient characteristics
The mean age at the time of enrollment was 44 years (range, 29–58 years). Eighteen patients (90%) had clinical T2 or T3 tumors at diagnosis. LN status was evaluated before NAC using FNA in ten patients and by core needle biopsy in ten patients. Nine patients had pathologically negative but clinically suspicious LN metastasis on US or PET-CT. The remaining 11 patients had pathologically confirmed metastatic marker-clipped nodes. The clinicopathological characteristics of the patients are summarized in Table 1.
Clip insertion and wire localization
A total of 24 clips were inserted in 20 patients. One marker clip was inserted in 16 patients, and two marker clips were inserted in four patients (cases #6, 9, 10 and 11). The day after the clip insertions, five clips were not found by MLO view in three patients. The day before surgery, seven clips were not found by MLO view in five patients. Instead, their locations were confirmed by fluoroscopic-guided imaging. Intraoperatively, all seven clips were placed in deep locations of axillary level I or II LNs. Wire localization using a CBCT system was successfully performed in all 24 clips, but one clip could not be retrieved. In this case, two clips were inserted in the same patient (case #11). Localization was performed with two wires in axillary level I and II marker-clipped LNs. However, the level II marker clip could not be found and retrieved intraoperatively, possibly due to loosening of the anchored hook. In order not to leave metastatic residues in this patient, we performed full ALND until level II. The location of the clip that we failed to retrieve was confirmed on the 6-month follow-up chest CT at the placement site, without migration.
Surgical procedure and pathologic outcomes
Table 2 lists the responses of the primary tumor and axillary LNs after NAC. A total of 24 marker clips were inserted, and 23 marker-clipped LNs were successfully retrieved during surgery (identification rate, 23/24, 95.8%). The mean number of retrieved SLNs was 2.26 (range, 1–7). Seven clipped LNs were placed in deep locations of axillary level I or II LNs; the remaining 17 clipped LNs were matched with the SLNs. pCR was defined as the absence of invasive or noninvasive residual tumor in the breast or lymph nodes (ypT0ypN0) upon pathological examination [16].
In the 11 patients with metastatic marker-clipped LNs, four patients converted to negative status after NAC, and their SLNs were all also negative. Four patients (4/11, 36.4%) achieved pCR in the axillary LNs. The other seven patients maintained metastasis in the marker-clipped LNs. Of these seven patients, five underwent ALND. Three of the seven patients had metastatic residues in the ALNs, and two of the three patients also had negative results in the SLNs; the marker-clipped nodes accurately predicted axillary nodal status in these two patients. Nine patients had pathologically negative marker-clipped LNs initially. All marker-clipped LNs remained negative after NAC.
All patients underwent follow-up examinations for axillary recurrence until June 2017, with a mean follow-up period of 15.3 months. Postoperative mammography, US, serum tumor markers and clinical breast examinations were performed every 6 months. Breast MRI, whole-body bone scans and chest CTs, serum tumor markers and clinical breast examinations were performed annually. Disease-free status of the axilla was confirmed in all 20 patients.
Twelve patients did not undergo ALND. Instead, axillary recurrence was evaluated, and all patients were confirmed to have a disease-free status in the axilla. Eight of the 12 patients without ALND underwent radiation therapy of the regional LNs (supraclavicular fossa, internal mammary chain and axillary bed).
There were no complications such as bleeding, hematoma formation or nerve injury during clip insertion or localization. No intraoperative or postoperative complications were reported. The 20 patients’ clinicopathological stages and pathological status of the axillary LNs before and after NAC are summarized in Table 3.
Discussion
The aim of this study was to assess the feasibility and accuracy of marker-clip placement and SLNB for evaluation of the axillary response to NAC. Marker-clip placement was successful in all patients and was easily performed [13]. Selective removal of marker-clipped nodes with SLNB may improve the accuracy of residual disease evaluation in the axilla and help identify candidates for limited axillary surgery after NAC.
Of the 11 patients with pathologically confirmed metastatic lesions before NAC, seven had residual disease identified in the marker-clip node. Three of these seven patients had metastatic residues on the ALNs, and two of the three patients with metastatic residues on the ALNs had even negative results in the SLNs. This suggests that marker-clip localization detected two cases of false-negative ALNs if we only performed SLNB.
Twelve patients did not undergo ALND. Instead, axillary recurrence was evaluated, and all patients were confirmed to have a disease-free status in the axilla. Eight of the 12 patients without ALND underwent radiation therapy in the regional LNs (supraclavicular fossa, internal mammary chain and axillary bed) to prevent axillary recurrence according to the NCCN guidelines [12]. If clinicians are reluctant to omit ALND in certain patients, radiation therapy may be an alternative. The Alliance A11202 study and the NSABP B-51/Radiation Therapy Oncology Group (RTOG) 1304 randomized phase 3 trial will clarify the role of locoregional radiotherapy for patients with clinical N1 disease that becomes node-negative after NAC.
The feasibility and accuracy of SLNB after NAC had been questioned since chemotherapy might alter lymphatic drainage patterns, thereby affecting the identification rate and accuracy of lymphatic mapping. Furthermore, false-negative results can occur when metastases are not found in SLNs but are found in non-sentinel or axillary nodes. The FNR of SLN surgery after NAC was approximately 12% in a meta-analysis [17,18,19]. However, several approaches have been proposed to omit ALND after NAC, thereby minimizing morbidity from ALND. Moreover, the axillary pCR rate ranges from 22 to 52%, and this result has implications for SLNB as a potential alternative to ALND in patients treated with NAC [2, 20, 21]. To decrease the likelihood of residual metastasis in non-SLNs, TAD with clips or radioactive seed placements in positive LNs has been proposed [10, 11, 22]. A recent study reported a minimal acceptable FNR of 10% [23]. The identification rate and FNR of targeted nodes ranged from 60–97% to 4–10%, respectively, in three studies [12, 21, 23]. We also demonstrated a very good identification rate of 95.8%.
The “Marking Axillary Lymph Nodes with the Radioactive Iodine 125I Seeds” (MARI) procedure stated that marking and selectively removing metastatic LNs after NAC led to an identification rate of 97% and FNR of 7% [22]. The ACOSOG Z1071 trial, with 170 (83.7%) patients with clinical N1 disease and at least two resected SLNs, reported that clip location was confirmed in 141 cases and that the FNR was 6.8% [11]. The MD Anderson Cancer Center also presented a prospective study on TAD in which marker-clipped nodes revealed metastases in 115 patients, resulting in an FNR of 4.2% [24].
The ACOSOG Z1071, SN FNAC and SENTINA trials recommended removing more than two SLNs to lower the FNR to approximately 10%. The St. Gallen consensus also stated that SLNB was appropriate only in cases where three or more sentinel nodes were examined after NAC [25]. Since the mean number of retrieved SLNs in our study was 2.26, the surgical extent of our SLNB was acceptable compared to recent studies [5].
The procedures carried out in the present study were technically feasible and safe—we did not observe complications such as bleeding or hematoma. However, the study had several limitations as well as strengths. We could not report the FNR because 12 patients did not undergo ALND. However, it was ethically and practically feasible and safe to omit ALND for several reasons. First, the mean number of retrieved SLNs was 2.26. Second, most previous trials have yielded similar results regarding the accuracy of SLNB in a neoadjuvant setting. Finally, postoperative mammography, US and MRI at 6 months confirmed the disease-free status of the axilla in these 12 patients.
The second limitation is that we inserted the clip based on suspicious findings by US or PET-CT rather than on biopsy results. However, we found one false-negative case after FNA. One patient (case #4) had a negative result upon initial FNA. However, after re-evaluation of the axilla, the core needle biopsy revealed metastasis. This result implies that FNA may not be an entirely reliable method for evaluating LN metastasis. The SENTINA study also assessed axillary status clinically by palpation or US but not pathologically [10]. Pathological confirmation of clinically suspicious LNs will be necessary in future studies.
The final limitation is the extra radiation caused by several procedures. We used a fluoroscopic method instead of US to detect the marker clips before surgery. Other than fluoroscopy, the unilateral MLO mammography that was performed one day after the clipping and one day before the surgery, as well as the preoperative CBCT, introduced extra radiation. One radiation dose of unilateral MLO mammography was 0.4 mGy. CBCT was performed twice to locate the clip first and then to locate both the marker clip and needle tip. The radiation dose for each CBCT was 20 mGy. The radiation dose using fluoroscopy was 1.6 mGy per 3 s. Each patient was exposed to fluoroscopy for 30 s, which means that each patient was exposed to 16 mGy for 30 s. In total, each patient was exposed to 56.8 mGy during these procedures. According to the International Commission on Radiological Protection (ICRP) recommendations, doses under 50–60 mSv per year are allowable [26]. Since 1 mSv is the dose produced by exposure from 1 mGy, 56.8 mGy is an acceptable dose.
In conclusion, image-guided marker-clip placement on positive ALNs in breast cancer before NAC and removal with SLNB is technically feasible. This procedure can improve the accuracy of the residual disease evaluation of the axilla, especially in patients who are negative upon SLNB, and can help identify candidates for limited axillary surgery after NAC. This is a promising method, and further studies are needed to evaluate the oncologic safety of this procedure in patients who do not undergo ALND.
References
Fisher B, Brown A, Mamounas E et al (1997) Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from national surgical adjuvant breast and bowel project B-18. J Clin Oncol 15:2483–2493
Kuerer HM, Sahin AA, Hunt KK et al (1999) Incidence and impact of documented eradication of breast cancer axillary lymph node metastases before surgery in patients treated with neoadjuvant chemotherapy. Ann Surg 230:72–78
Fu JF, Chen HL, Yang J et al (2014) Feasibility and accuracy of sentinel lymph node biopsy in clinically node-positive breast cancer after neoadjuvant chemotherapy: a meta-analysis. PLoS ONE 9:e105316
Mamounas EP, Brown A, Anderson S et al (2005) Sentinel node biopsy after neoadjuvant chemotherapy in breast cancer: results from national surgical adjuvant breast and bowel project protocol B-27. J Clin Oncol 23:2694–2702
Shen J, Gilcrease MZ, Babiera GV et al (2007) Feasibility and accuracy of sentinel lymph node biopsy after preoperative chemotherapy in breast cancer patients with documented axillary metastases. Cancer 109:1255–1263
Hieken TJ, Boughey JC, Jones KN et al (2013) Imaging response and residual metastatic axillary lymph node disease after neoadjuvant chemotherapy for primary breast cancer. Ann Surg Oncol 20:3199–3204
Straver ME, Loo CE, Alderliesten T et al (2010) Marking the axilla with radioactive iodine seeds (MARI procedure) may reduce the need for axillary dissection after neoadjuvant chemotherapy for breast cancer. Br J Surg 97:1226–1231
Boughey JC, Suman VJ, Mittendorf EA et al (2013) Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA 310:1455–1461
Boileau JF, Poirier B, Basik M et al (2015) Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol 33:258–264
Kuehn T, Bauerfeind I, Fehm T et al (2013) Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol 14:609–618
Boughey JC, Ballman KV, Le-Petross HT et al (2016) Identification and resection of clipped node decreases the false-negative rate of sentinel lymph node surgery in patients presenting with node-positive breast cancer (T0-T4, N1-N2) who receive neoadjuvant chemotherapy: results from ACOSOG Z1071 (Alliance). Ann Surg 263:802–807
National Comprehensive Cancer Network NCCN (2016) Clinical practice guidelines in oncology, version 2. National Comprehensive Cancer, Network, Washington
Youn I, Choi SH, Kook SH et al (2015) Ultrasonography-guided surgical clip placement for tumor localization in patients undergoing neoadjuvant chemotherapy for breast cancer. J Breast Cancer 18:44–49
Giuliano AE, Hunt KK, Ballman KV et al (2011) Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 305:569–575
Edge SB, Compton CC (2010) The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17:1471–1474
Sinn HP, Schmid H, Junkermann H et al (1994) Histologic regression of breast cancer after primary (neoadjuvant) chemotherapy. Geburtshilfe Frauenheilkd 54:552–558
Xing Y, Foy M, Cox DD et al (2006) Meta-analysis of sentinel lymph node biopsy after preoperative chemotherapy in patients with breast cancer. Br J Surg 93:539–546
Pilewskie M, Morrow M (2017) Axillary nodal management following neoadjuvant chemotherapy: a review. JAMA Oncol 3:549–555
El Hage Chehade H, Headon H, Kasem A et al (2016) Refining the performance of sentinel lymph node biopsy post-neoadjuvant chemotherapy in patients with pathologically proven pre-treatment node-positive breast cancer: an update for clinical practice. Anticancer Res 36:1461–1471
Hennessy BT, Hortobagyi GN, Rouzier R et al (2005) Outcome after pathologic complete eradication of cytologically proven breast cancer axillary node metastases following primary chemotherapy. J Clin Oncol 23:9304–9311
Enokido K, Watanabe C, Nakamura S et al (2016) Sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with an initial diagnosis of cytology-proven lymph node-positive breast cancer. Clin Breast Cancer 16:299–304
Donker M, Straver ME, Wesseling J et al (2015) Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: the MARI procedure. Ann Surg 261:378–382
Patten DK, Zacharioudakis KE, Chauhan H et al (2015) Sentinel lymph node biopsy after neo-adjuvant chemotherapy in patients with breast cancer: are the current false negative rates acceptable? Breast 24:318–320
Caudle AS, Yang WT, Krishnamurthy S et al (2016) Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol 34:1072–1078
Coates AS, Winer EP, Goldhirsch A et al (2015) Tailoring therapies–improving the management of early breast cancer: st Gallen international expert consensus on the primary therapy of early breast cancer 2015. Ann Oncol 26:1533–1546
ICRP (2007) The 2007 recommendations of the international commission on radiological protection. ICRP publication 103. Ann ICRP 37:1–332
Acknowledgements
This work was supported by a Samsung Biomedical Research Institute (SBRI) grant.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare. This manuscript has not been published elsewhere.
Rights and permissions
About this article
Cite this article
Kim, E.Y., Byon, W.S., Lee, K.H. et al. Feasibility of Preoperative Axillary Lymph Node Marking with a Clip in Breast Cancer Patients Before Neoadjuvant Chemotherapy: A Preliminary Study. World J Surg 42, 582–589 (2018). https://doi.org/10.1007/s00268-017-4171-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-017-4171-8