Abstract
Background
Identification and resection of a clipped node was shown to decrease the false-negative rate (FNR) of sentinel lymph node biopsy (SLNB) after neoadjuvant chemotherapy (NAC) for patients presenting with initially node-positive breast cancer.
Methods
Between March 2014 and March 2016, a prospective trial analyzed 98 patients with axilla-positive locally advanced breast cancer (T1-4, N1-3) to assess the feasibility and efficacy of placing clips into most suspicious biopsy-proven node. The study considered blue, radioisotope active, and suspiciously palpable nodes as sentinel lymph nodes (SLNs).
Results
The SLN identification rate was 87.8%. The median age of the patients with an SLNB (n = 86) was 44 years (range 28–66 years). Of these patients, 77 (88.4%) had cT1-3 disease, and 10 (11.6%) had cT4 disease. The majority of the patients (n = 66, 76.7%) had cN1, whereas 21 patients (23.3%) had cN2 and cN3. A combined method was used for 37 patients (43%), whereas blue dye alone was used for the remaining patients (57%). The clipped node was the SLN in 70 patients (81.4%). For the patients with cN1 before NAC, the FNR was found to be 4.2% (1/24) when the clipped node was identified as an SLN. However, the FNR was estimated to be as high as 16.7% (1/6) for the patients with cN1 before NAC when the clipped node was found to be a non-SLN.
Conclusions
The study results also suggest that axillary dissection could be omitted for patients presenting initially with N1 disease and with a negative clipped node as the SLN after NAC due to the low FNR.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The presence of axillary metastases is an important prognostic factor that affects treatment decisions in breast cancer. Neoadjuvant chemotherapy (NAC) has become the standard of care for the treatment of patients with clinically node-positive breast cancer and can eradicate axillary metastasis in 40–75% of patients, depending on tumor subtype.1,2,3,–4 This causes interest in avoiding extensive surgery when NAC eradicates metastasis in lymph nodes.
The therapeutic effect of an axillary lymph node dissection (ALND) is limited in the complete pathologic response in the axilla. The American College of Surgeons Oncology Group (ACOSOG) Z1071 trial and the European SENTinel NeoAdjuvant (SENTINA) trial reshaped surgical management via considering axillary staging with sentinel lymph node biopsy (SLNB) after NAC for node-positive patients.5,6 These trials were designed to determine whether SLNB is accurate in staging the axilla after NAC for patients with known axillary metastasis. Although SLNB after NAC is a less invasive method for restaging axillary disease, it has been related to a higher false-negative rate (FNR).
Placement of a clip in the cytopathologically proven positive node before NAC is one method that can help to remove an initially biopsy-proven positive node at the time of surgery. The FNR is reduced as a result of removing the clipped node as the sentinel lymph node (SLN).7
This study aimed to assess the feasibility and efficacy of placing clips in the most suspicious biopsy-proven node to reduce the FNR of SLNB after NAC at our institution.
Materials and Methods
Between March 2014 and March 2016, a prospective registry trial was performed with patients who had clinical axilla-positive locally advanced breast cancer (cT1-4, cN1-3) at the Department of Surgery, Istanbul Faculty of Medicine, University of Istanbul. The study was approved by the Istanbul University Ethics Commitee, and all the participating patients gave informed consent.
Patients with any clinical positive axilla were included in the study. The staging criteria of the American Joint Committee on Cancer (AJCC) 7th edition8 were used in the clinical and pathologic evaluation of the patients. Those younger than 18 years and those 70 years of age or older with inflammatory breast cancer, previous axillary surgery or pregnancy, or symptomatic distant metastases were not eligible for inclusion in the study.
Patient Evaluation
All the patients who had clinical locally advanced breast cancer with ipsilateral suspicious axillary lymph nodes underwent routine breast imaging including breast ultrasound, mammography, and magnetic resonance imaging (MRI) at our institution. Diagnosis of the primary breast tumors was performed with a core biopsy, and the patients were evaluated with [18F]-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET)/computed tomography (CT) for detection of distant metastases.
Axillary Ultrasound and Clip Placement Procedure
The clinically positive axillary lymph node or nodes at diagnosis were confirmed by cytopathologic analysis of ultrasound-guided fine-needle aspiration biopsy material for all the patients. Before NAC was started, commercially available titantium clips (766914100SST V Mark Breast Biopsy Site Marker, 14 G; Argon Medical Devices, Athens, TX, USA) were used to mark the most suspicious axillary lymph node as the index node under ultrasound guidance by two dedicated breast radiologists (D.K., G.E.) at the Institute of Oncology, University of Istanbul. The index node was found by using one or more of the following criteria as described previously:9 asymmetric cortical thickening (≥ 3 mm), loss of fatty hilum, abnormal lymph node shape (round form), cortical abnormalities including cortical heterogenous/multilobulations, and increased peripheral blood flow.
Preoperative Chemotherapy
The patients received NAC as a regimen containing four cycles of adriamycine with cyclophosphamide (AC) followed by weekly taxan for 12 weeks. Trastuzumab was added to weekly taxanes for patients with human epidermal growth factor receptor 2 (HER2)-neu overexpression.
Surgical Procedure and SLNB Technique
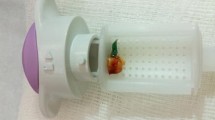
In 2–3 weeks after completion of the NAC, the patients were clinically evaluated by physical examination together with repeat mammography and breast ultrasonography and breast MRI to assess the NAC response. Biopsy of the SLN was performed only for patients with a clinical negative axilla determined by physical exam and radiologic imaging including mainly ultrasound and MRI findings. Lymphatic mapping was performed, followed by peritumoral and/or subareoler injection of isosulphan blue dye alone or in addition to a technetium Tc 99 m sulfur colloid as described previously.10 All blue, radioisotope-active, and suspicious palpable nodes were removed as SLNs, and the patients subsequently underwent completion of ALND. Specimen radiographs were obtained for the dissected SLNs and ALND for visualization of the clipped node (Fig. 1).
Pathologic Evaluation
The sentinel lymph nodes were examined as described previously.11 Briefly, at least four sections were obtained from each block of a sentinel node at 250-µm intervals and stained with hematoxylin and eosin (H&E). Sentinel lymph nodes containing micrometastases or isolated tumor cells detected by H&E or cytokeratin immunochemistry (IHC) staining were considered positive according to the AJCC 7th edition. Non-SLNs were evaluated only by H&E staining, and pathologic findings regarding chemotherapy response also were recorded including regressional fibrosis, fibrohyalinization, reactive changes, and metastases. Pathologic complete response (pCR) was defined as the complete eradication of all invasive and noninvasive cancer.12
Statistical Analysis
Data were analyzed by using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). Descriptive analyses were used to calculate the SLN identification rate and the clipped node identification rate among SLNs. Furthermore, 2 × 2 contingency tables were constructed to determine the FNRs for different parameters. A successfully mapped SLN was described as a false-negative SLN when routine pathologic analysis with H&E staining and IHC failed to detect tumor cells in the SLN during detection of tumor cells in non-SLNs obtained in ALND. The Mann-Whitney U test was used for continuous variables to compare the ultrasound findings of the index node associated with cN2 and cN3 disease with cN1 disease, and Fisher’s exact test was used for categorical variables. A p value lower than 0.05 was considered significant, and factors found to be significant in univariate analyses were further analyzed via forward logistic regression analysis.
Results
Of the 105 patients, 6 (5.7%) were found to be axilla-positive in the clinical evaluation, which included physical exam and radiology in ultrasound and/or MRI imaging after NAC, and were excluded from the study. In 3–5 weeks after completion of NAC, the patients were scheduled for surgery and an SLNB was performed for the remaining 99 patients, who were thought more likely to be clinically axilla-negative. Of these patients, 87 had a successful mapping, with an identification rate of 87.8%, whereas one patient was further excluded from the study because of an SLN found to be a mammaria interna lymph node under gamma probe guidance without any axillary SLN.
The patient and tumor characteristics are shown in Table 1. Briefly, the median age was 44 years (range 28–66 years). Of those with an axillary SLNB, 77 had cT1-3 disease (88.4%), and 10 had cT4 disease (11.6%). The majority of the patients had cN1 disease (n = 66, 76.7%), whereas 21 patients (23.3%) had cN2 or cN3 disease. The combined method was used for 37 patients (43%), whereas blue dye alone was used for the remaining patients (57%). The median number of SLNs was two (range 1–7). The majority of the patients (67.4%) had two or more SLNs identified.
Interestingly, the clipped node could not be found among the SLNs or in the axillary lymph node specimen of three patients (3.5%). In one of these patients, clip migration to the fatty tissue was detected postoperatively by radiologic imaging (Fig. 2). In 70 (81.4%) of 86 patients, the clipped node was the SLN, whereas in the remaining 16 patients (18.6%), the clipped node was the non-SLN. In this study, ALND was omitted for five patients with a clipped node as the SLN and a negative intraoperative pathologic examination. The FNR estimations were therefore performed for the remaining 81 patients with ALND.
In our study, the overall FNR was 11.4%. The FNRs were analyzed according to the patient and SLN characteristics as shown in Table 2. Briefly, among the patients with cN1 before NAC, the FNR was found to be 4.2% (1/24) when the clipped node was identified as the SLN. However, the FNR was estimated to be as high as 16.7% (1/6) among the patients with cN1 before NAC when the clipped node was detected as the non-SLN. Furthermore, the FNRs were found to be improved in patients with cT1-3 and cN1 disease, using combined technique, with excision of two or more SLNs and with a breast pCR.
The pathologic examination showed pCR in the breast and axilla of 22 patients (26.2%), whereas nodal pCR was found in 29 patients (33.7%) together with a breast pCR rate of 31% in our series. Notably, the patients with a breast pCR had an FNR of 0% in the entire cohort.
Ultrasonographic abnormalities of the clipped index lymph node (CILN) were analyzed by comparing the patients who had cN1 with those who had cN2 (Table 3). The median lymph node size was 21.5 mm (range 9–39 mm). The majority of the patients with cN1 (70%) had three or fewer suspicious LNs detected by ultrasound. The significant factors distinguishing CILN in cN2 and cN3 from that in cN1 were found to be tumor size 2.5 cm or larger, cortical thickness of 1 cm or more, and increased peripheral blood flow. Multivariate logistic regression analysis for predicting factors in ultrasound findings of the CILN associated with cN2 and cN3 (vs. cN1) identified lymph node size of 2.5 cm or larger (odds ratio [OR], 24.41; 95% confidence interval [CI], 3–199.8; p = 0.003) and the presence of peripheric blood flow (OR 14.21; 95% CI 1.05–193.3; p = 0.046).
Discussion
The accuracy and oncologic safety of SLNB procedure in patients with cN+ locally advanced breast cancer is an ongoing concern. Both the ACOSOG Z1071 and SENTINA trials investigated the role of SLNB after downstaging of the axilla with NAC. Both studies found that as the number of sentinel nodes removed increases, the FNR decreases, and at least two or three nodes should to be taken as SLNs.5 The ACOSOG Z1071 trial evaluated the FNR of SLN surgery for patients with clinical T0-4, N1-2 disease treated with NAC and found that the FNR was 12.6% for N1 patients with two or more SLNs resected.5 Furthermore, the FNR decreased to 9.1% when surgeons identified three SLNs in addition to using radiolabeled colloids with blue dye. The Z1071 trial showed that an acceptable FNR could be obtained with SLNB after NAC for biopsy-proven N1 or N2 patients. Similar results were published in the SENTINA trial, corroborating these findings showing an overall FNR of 14.2%.6
In the current study, we report an overall FNR of 11.4% for the patients who presented with node-positive cT1-4/cN1-3 disease and received NAC after placement of clips into the metastatic node. This FNR seems to be better than in the randomized trials, SENTINA and Z1071, with patient accrual from more than 100 centers, but similar to the FNR in single-institution series, with the MD Anderson Cancer Center showing an FNR of 10.1%, as reported by Caudle et al.13 In concordance with the SENTINA and Z1071 trials, use of combined technique or excision of two or more SLNs reduced the FNR to 0% for cN1 patients in our series.
A subset analysis of the Z1071 trial remarkably showed that FNR further decreased to 6.8% for those patients with placement of a clip in metastatic nodes when the clipped node was in the SLN specimen.7 In contrast, the FNR was 19% when the clipped node was in the ALND specimen. After this study, Caudle et al.13 at the MD Anderson Cancer Center initiated an approach called targeted axillary dissection (TAD) by placing a clip into the metastatic lymph node before starting chemotherapy and removing those clipped nodes with wire or radioactive seed localization after completion of NAC.14 They tested the feasibility of this procedure and demonstrated that axillary clipped lymph nodes could be localized and removed after placement of radioactive iodine (I)-125-labeled seeds.15
Furthermore, Caudle et al.13 subsequently published their experience with TAD, reporting an FNR of 4.2% with removal the clipped node alone.13 İn their series, the clipped node was not retrieved as an SLN in 23% of the cases, and removal of the clipped node along with the SLNs further reduced the FNR to 2%. Our goal in this trial was to demonstrate the feasibility and efficacy of retrieving the clipped node as an SLN compared with other factors affecting FNR at our institution after NAC. In concordance with the findings by Caudle et al.,13 the clipped node could not be detected among SLNs in almost 19% of the patients, and the FNR was similarly 4.2% for cases in which the clipped node was found among SLNs in specimen radiographs.
Our results demonstrate that marking suspicious nodes with clips before NAC seems to be a feasible technique because the clipped nodes could be retrieved as SLNs or non-SLNs in almost all patients, in concordance with other reports.16,17 The FNR also was found to be increased, reaching 16.7% for those in whom the clipped node could not be found among SLNs. This is comparable with the Z1071 subset analysis, which reported an FNR of 19% when the clipped node was found in the ALND specimen.
In this study, we also described the ultrasound findings of the clipped index lymph node. The subject by which the lymph node should be considered as the index node to be marked if more than one suspicious lymph node exists is under discussion, and the criteria may vary among radiologists because ultrasound is an operator-dependent procedure. The following abnormal features were suggested9: asymmetric cortical thickening/lobulations, loss or compression of the hyperechoic medullary region, absence of fatty hilum, abnormal lymph node shape, hypoechoic cortex, admixture of normal- and abnormal-appearing nodes, and increased peripheral blood flow. Loss or compression of the hyperechoic medullary region, absence of fatty hilum, abnormal lymph node shape, and increased peripheral blood flow were found to be predictors of N2-3 disease. Our results similarly show that the significant factors distinguishing CILN in cN2 and cN3 from cN1 were lymph node size of 2.5 cm or larger and increased peripheral blood flow. Therefore, other abnormal features also should be taken into consideration together with the size of the abnormal lymph node in determining the index node. Furthermore, in trials regarding TAD, it is highly recommended that patients with more than three suspicious lymph nodes not be included.
Because the number of patients with cT4 or cN2/N3 was limited in our series, it may be difficult to comment on the unacceptable high rates of FNR in these subpopulations. However, the patients with breast pCR were found to have extremely low FNR, reaching 0%, regardless of cN status, suggesting that those who have an excellent chemotherapy response with cN2 could still be candidates for SLNB, thus warranting more studies in patients with cN2-3. Tadros et al.18 have shown an increased relative risk of nodal metastases, reaching 7.4 (95% CI 3.7–14.8; p < 0.001) for patients with a partial breast response versus patients with pCR among those who have triple-negative or HER2-neu (+) disease with a high likelihood of pCR.
Based on the findings of this study, feasibility clinical trials are ongoing around the world to determine whether a nonoperative management can be considered for those selected cT1-3/cN0-3 patients with triple negativity or HER2-neu positivity who have a breast pCR as determined by a negative vacuum biopsy from the tumor bed.19
In conclusion, our results suggest that axillary dissection could be omitted for patients who present initially with N1 disease and a negative clipped node as the SLN after NAC due to the low FNR. Targeted axillary dissection may be required for patients with a clipped node as the non-SLN in addition to SLNB.
References
Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–85.
Dominici LS, Negron Gonzalez VM, Buzdar AU, et al. Cytologically proven axillary lymph node metastases are eradicated in patients receiving preoperative chemotherapy with concurrent trastuzumab for HER2-positive breast cancer. Cancer. 2010;116:2884–9.
Hennessy BT, Hortobagyi GN, Rouzier R, et al. Outcome after pathologic complete eradication of cytologically proven breast cancer axillary node metastases following primary chemotherapy. J Clin Oncol. 2015;23:9304–11.
Alvarado R, Yi M, Le-Petross H, et al. The role for sentinel lymph node dissection after neoadjuvant chemotherapy in patients who present with node- positive breast cancer. Ann Surg Oncol. 2012;19:3177–84.
Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOGZ1071 (Alliance) clinical trial. JAMA. 2013;310:1455–61.
Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel lymph node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14:609–18.
Boughey JC, Ballman KV, Le-Petross HT, et al. Identification and resection of the clipped node decreases the false-negative rate of sentinel lymph node surgery in patients presenting with node positive breast cancer (T0-4, N1-2) who receive neoadjuvant chemotherapy-results from ACOSOG Z1071 (Alliance). Ann Surg. 2016;263:802–7.
AJCC. Cancer staging handbook. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A (eds) AJCC Cancer Staging Manual, 7th ed. Springer, New York, 2010.
Moore A, Hester M, Nam MW, et al. Distinct lymph nodal sonographic characteristics in breast cancer patients at high risk for axillary metastases correlate with the final axillary stage. Br J Radiol. 2008;81:630–6.
Eroglu A, Mudun A, Ozmen V, et al. Comparison of subdermal and peritumoral injection techniques of lymphoscintigraphy to determine the sentinel lymph node in breast cancer. Clin Nucl Med. 2004;29:306–11.
Ozmen V, Unal ES, Muslumanoglu ME, et al. Axillary sentinel node biopsy after neoadjuvant chemotherapy. Eur J Surg Oncol. 2010;36:23–9.
von Minckwitz G, Rezai M, Loibl S, et al: Capecitabine in addition to anthracycline/taxane based neoadjuvant treatment in patients with primary breast cancer: the phase III GeparQuattro study. J Clin Oncol. 2010;28:2015–23.
Caudle AS, Yang WT, Krishnamurthy S, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. 2016;34:1072–8.
Mittendorf EA, Caudle AS, Yang W, et al. Implementation of the American College of Surgeons Oncology Group Z1071 trial data in clinical practice: is there a way forward for sentinel lymph node dissection in clinically node-positive breast cancer patients treated with neoadjuvant chemotherapy? Ann Surg Oncol. 2014;21:2468–73.
Caudle AS, Yang WT, Mittendorf EA, et al. Selective surgical localization of axillary lymph nodes containing metastases in patients with breast cancer: a prospective feasibility trial. JAMA Surg. 2015;150:137–43.
Diego EJ, McAuliffe PF, Soran A, et al. Axillary staging after neoadjuvant chemotherapy for breast cancer: a pilot study combining sentinel lymph node biopsy with radioactive seed localization of pretreatment positive axillary lymph nodes. Ann Surg Oncol. 2016;23:1549–53.
Kim EY, Byon WS, Lee KH, et al. Feasibility of preoperative axillary lymph node marking with a clip in breast cancer patients before neoadjuvant chemotherapy: a preliminary study. World J Surg. 2018;42:582–9.
Tadros AB, Yang WT, Krishnamurthy S, et al. Identification of patients with documented pathologic complete response in the breast after neoadjuvant chemotherapy for omission of axillary surgery. JAMA Surg. 2017;152:665–70.
Rauch GM, Kuerer HM, Adrada B, et al. Biopsy feasibility trial for breast cancer pathologic complete response detection after neoadjuvant chemotherapy: imaging assessment and correlation end points. Ann Surg Oncol. 2018;25:1953–60.
Acknowlegment
The study has been financially supported by the Istanbul Breast Society. The authors also thank Çiğdem Kanbesler for her secterial assistance in data retrieval.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cabıoğlu, N., Karanlık, H., Kangal, D. et al. Improved False-Negative Rates with Intraoperative Identification of Clipped Nodes in Patients Undergoing Sentinel Lymph Node Biopsy After Neoadjuvant Chemotherapy. Ann Surg Oncol 25, 3030–3036 (2018). https://doi.org/10.1245/s10434-018-6575-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-018-6575-6