Abstract
Background
Postoperative cholangitis is one of the major late complications after pancreaticoduodenectomy (PD), and recurrent cholangitis has a negative impact on patients’ quality of life. However, detailed reports are scarce. The aim of this study was to investigate the clinical features of postoperative cholangitis after PD.
Methods
Between January 2007 and December 2013, 155 consecutive patients underwent PD. Of these, 113 patients were included in this study. Cholangitis was diagnosed according to the criteria in the revised Tokyo Guidelines 2013, and repeated cholangitis with three or more episodes was defined as ‘refractory cholangitis’. Data from patients with refractory cholangitis were retrospectively analyzed.
Results
Refractory cholangitis was observed in 21 patients (18.6%). Of these, 17 patients experienced cholangitis within 1 year after PD, and 10 patients had biliary strictures. These patients required an average of two interventional or endoscopic treatments for stricture dilatation, which led to remission. The 2-year cumulative incidence rate for refractory cholangitis was 18.9% (95% CI 11.65–26.15). Multivariate analysis revealed five risk factors for developing refractory cholangitis: benign disease (odds ratio [OR] 18.52; P = 0.001), long operation time (OR 18.73; P = 0.002), elevated C-reactive protein (OR 6.55; P = 0.014), elevated alkaline phosphatase (OR 6.03; P = 0.018), and the presence of pneumobilia (OR 28.81; P = 0.009).
Conclusions
Postoperative refractory cholangitis after PD usually developed within a year. Almost half of the patients had biliary strictures, and aggressive dilatation might be effective to achieve remission in these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreaticoduodenectomy (PD) is a high-risk surgical procedure that is primarily indicated for carcinomas of the pancreatic head or periampullary region. Due to advances in surgical techniques, perioperative management, and interventional radiology, postoperative mortality associated with PD has declined; however, postoperative morbidity remains as high as 50%, even in specialized pancreatic units [1,2,3,4,5,6].
The prognosis for patients with pancreatic adenocarcinoma has improved over recent years, and long-term survival after PD is increasing. In addition, the indication for PD has been extended to low-grade malignancies (e.g., intraductal papillary mucinous neoplasms) [3,4,5], which has led to an increase in hepaticojejunostomy of non-dilated bile ducts. However, the majority of studies on morbidity after PD have focused on short-term surgical outcomes, and only a few studies have reported on long-term outcomes such as hepatic steatosis or cholangitis [3, 7,8,9,10,11,12].
Postoperative cholangitis, which is thought to be caused by anastomotic strictures, is one of the major late complications after PD [7, 10, 11]. Although cholangitis has a negative impact on quality of life, long-term clinical outcomes have not been thoroughly investigated. Furthermore, the definition of cholangitis after PD is not unified, making it more difficult to discuss disease management. Clinical features, including the frequency and timing of episodes, and treatment as well as risk factors for postoperative cholangitis after PD remain to be clarified. The aim of this study was to evaluate the clinical features of postoperative cholangitis after PD.
Methods
Patients
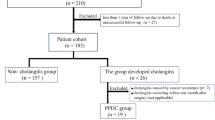
Between January 2007 and December 2013, 155 consecutive patients underwent PD at Tokyo Medical and Dental University Hospital. Of these, 113 patients with at least 1 year of postoperative follow-up were included in this study. Exclusion criteria were as follows: death within 1 year (21 patients), change of hospital (17 patients), prior choledochojejunostomy due to congenital choledochal cysts (2 patients), prior liver resection due to hepatocellular carcinoma and a liver abscess following transarterial chemoembolization (1 patient), and postoperative acquired hemophilia, a rare complication [13], with an atypical postoperative course (1 patient) (Fig. 1). Information was obtained from a prospective database combined with the hospital’s electronic patient record system. This retrospective, observational, single-center study was approved by the committee on life science and bioethics of Tokyo Medical and Dental University Hospital (Permission no. 2132).
Definition of cholangitis
Standard criteria for the diagnosis of acute cholangitis were first defined in the 2007 Tokyo Guidelines based on a literature review and an expert consensus at the International Consensus Meeting for the Management of Acute Cholecystitis and Cholangitis [14]. The Tokyo Guidelines were revised in 2013 (TG13) with new standard criteria for the diagnosis of acute cholangitis [15]. In the TG13, diagnosis of acute cholangitis consists of three categories: systemic inflammation (A), cholestasis (B), and imaging (C). Each category has 2 items as follows: category A, ‘fever and/or shaking/chills’ and ‘laboratory data: evidence of inflammatory response’; category B, ‘jaundice’ and ‘laboratory data: abnormal liver function’; and category C, ‘biliary dilatation’ and ‘evidence of the etiology on imaging’. To make a definitive diagnosis, one item from each of the three categories (A–C) is required [15]. In this study, postoperative cholangitis was diagnosed according to the TG13 criteria. ‘Refractory cholangitis’ was defined as at least three episodes of cholangitis after PD. Cholangitis due to tumor recurrence or metastasis was not included. According to House et al., biliary strictures were defined as radiologic-proven strictures at the level of the hepaticojejunostomy with obstructive jaundice in the setting of cholangitis [10]. All postoperative biliary strictures were confirmed by interventional radiology with percutaneous transhepatic cholangiography (PTC) or double balloon endoscopy (DBE). Patients without intrahepatic biliary tract dilatation were examined by magnetic resonance cholangiopancreatography (MRCP) to determine the presence of biliary strictures.
Surgical procedure
Most patients underwent subtotal stomach-preserving PD, in which the pylorus ring was resected while preserving more than 95% of the stomach. Conventional PD with gastrectomy or pylorus-preserving PD was occasionally performed. Reconstruction was mainly performed using the ante-colic Roux-en-Y method until 2011, and thereafter was changed to the modified Child’s method with Braun’s anastomosis [16]. Pancreaticojejunostomy was performed using the modified Blumgart method [17, 18]. Bile duct transection was made at the upper level of the junction of the cystic and common hepatic ducts, and hepaticojejunostomy was performed by single-layer, interrupted suture with an absorbable 4-0 or 5-0 thread. Stenting requirements for biliary anastomosis were decided by the surgeon on a case-by-case basis. All surgical procedures were performed by 10 pancreatic surgeons.
Early postoperative complications
Postoperative pancreatic fistula (POPF) was diagnosed according to the International Study Group on Pancreatic Fistula (ISGPF) guidelines [19]. Delayed gastric emptying (DGE) and postpancreatectomy hemorrhage (PPH) were diagnosed using the International Study Group of Pancreatic Surgery (ISGPS) guidelines [20, 21]. Bile leakage was diagnosed according to the International Study Group on Liver Surgery (ISGLS) guidelines [22]. These complications were considered to be clinically relevant if classified as grade B or C severity.
Follow-up after hospital discharge
Patients with an uneventful postoperative course were followed-up every 3–6 months, and blood tests and imaging studies were performed at least once every 6 months. As per clinical routine, patients with complications such as tumor recurrence were followed-up more regularly for testing and additional treatment. These laboratory data obtained after hospital discharge were analyzed.
Statistical analysis
The clinical characteristics of patients with and without refractory cholangitis were compared using the Chi-squared test, Fisher’s exact test, and the Mann–Whitney U test. Variables with a P value <0.05 were incorporated into a logistic regression model to examine the risk factors for refractory cholangitis. Risk factors are expressed as odds ratios (OR) with two-sided 95% confidence intervals (CI) and P values. Data are expressed as median values (range). P < 0.05 was considered to denote a statistically significant difference. All statistical analyses were performed using SPSS software version 23 (SPSS, Chicago, IL, USA).
Results
Patients’ characteristics
The clinical characteristics of patients are presented in Table 1. Among the 113 patients, the median age was 68 years (range 42–86 years), 73.5% were male, and the most common indication for PD was pancreatic adenocarcinoma (33.6%). The incidences of clinically relevant POPF, DGE, PPH, and bile leakage were 20.4, 15.9, 3.5, and 2.7%, respectively. The median length of follow-up was 40 months (range 12–99 months).
Refractory cholangitis after PD
The clinical characteristics of refractory cholangitis are presented in Table 2. Refractory cholangitis developed in 21 patients (18.6%). There were 143 episodes in all, and the median number of episodes was 5 (range 3–13 episodes) among these patients. Five patients (23.8%) developed more than 10 episodes. The median time to the first episode after PD was 7 months (range 2–23 months), and the first episode occurred within a year in 17 patients (81%). The median number of urgent admissions due to cholangitis was 2 (range 0–7).
Biliary strictures were found in 10 patients (by PTC in 5 and by DBE in 5). All 10 patients with strictures required radiologic or endoscopic treatment for dilatation, and 5 of the 10 patients had intrahepatic cholelithiasis. Patients with strictures required an average of 2 dilatations to achieve remission. None of the patients with strictures developed cholangitis after the last dilatation, although repeated dilatations were needed in some patients. The 11 patients without strictures were usually treated with pharmacological agents, most often with antibiotics. More patients with strictures required urgent admissions due to cholangitis than those without strictures (57 vs. 14%, P = 0.004). Despite pharmacotherapy, patients without strictures experienced recurrent cholangitis compared to patients with strictures (average interval of cholangitis: 5.3 and 8.3 months, respectively).
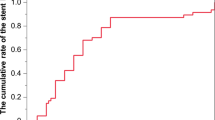
The cumulative incidence rate of refractory cholangitis after PD is shown in Fig. 2. The 1-year cumulative incidence rate was 15.0% (95% CI 8.33–21.67), and the 2-year incidence rate was 18.9% (95% CI 11.65–26.15).
Risk factors for refractory cholangitis
Risk factors for refractory cholangitis were divided into three categories: preoperative, operative, and postoperative factors. All factors were analyzed as univariate predictors (Table 3).
Univariate analysis of preoperative factors demonstrated that younger age and benign disease were risks for refractory cholangitis, whereas obstructive jaundice and biliary drainage were protective. Preoperative cholangitis and laboratory data were not predictive of refractory cholangitis.
Among the operative factors, operation time over 500 min correlated with refractory cholangitis. Estimated blood loss of >1000 mL tended to be more frequent in the refractory cholangitis group (P = 0.062). The reconstruction method, Roux-en-Y or modified Child’s method, did not influence the incidence of refractory cholangitis.
Analysis of postoperative factors demonstrated that refractory cholangitis was associated with clinically relevant POPF, elevated C-reactive protein (CRP) value before hospital discharge, elevated γ-glutamyltransferase (γ-GTP), and elevated alkaline phosphatase (ALP) after hospital discharge, and the presence of pneumobilia on imaging studies. Bile leaks after PD are uncommon [10]. We experienced bile leaks in only three patients (2.7%), and bile leaks were not predictive of refractory cholangitis.
Univariate analysis for refractory cholangitis identified ten significant risk factors: younger age, benign disease, without preoperative obstructive jaundice, without preoperative biliary drainage, long operation time, relevant POPF, perioperative CRP, postoperative γ-GTP and ALP, and the presence of pneumobilia.
Multivariate analysis revealed that risk factors for refractory cholangitis were: benign disease (OR 18.52; 95% CI 3.56–100; P = 0.001), long operation time (OR 18.73; 95% CI 3.07–114.38; P = 0.002), elevated CRP (OR 6.55; 95% CI 1.46–29.44; P = 0.0014), elevated ALP after discharge (OR 6.03; 95% CI 1.36–26.81; P = 0.018), and the presence of pneumobilia (OR 28.81; 95% CI 2.32–357.69; P = 0.009) (Table 4).
Discussion
Although postoperative cholangitis is one of the major late complications after PD, few data are available. This study evaluated the clinical characteristics of patients with refractory cholangitis after PD using the standardized definition for the diagnosis of cholangitis from the TG13.
The results demonstrated that 18.6% of patients developed refractory cholangitis and suffered several episodes and emergent hospitalizations. Most cases of refractory cholangitis developed within a year after PD. Postoperative refractory cholangitis is one of the most troublesome of the long-term complications after PD. In previous studies, frequencies of cholangitis after PD were 5% [3] and 6.7% [12], which were lower than in our study (18.6%). However, it is difficult to make between-study comparisons because of the lack of definitions of cholangitis in those reports. We hypothesized that there were more patients with benign disease and with intraductal papillary mucinous carcinoma (IPMC) who had a non-dilated common bile duct in our study (35.4%) than in those previous reports (25.2% [3] and 17.2% [12]) and that some of those patients developed cholangitis. Indeed, of 21 patients with refractory cholangitis, 16 patients (76%) had benign disease or IPMC.
Postoperative cholangitis after hepaticojejunostomy in other types of surgery is also a serious complication. Cholangitis has been reported to occur in 16.7% of patients after liver transplantation [23] and in 10% of patients after biliary tract surgery [24]. In these cases, anastomotic strictures after hepaticojejunostomy may be the primary cause of postoperative cholangitis. Studies suggested that the incidence of biliary strictures after PD ranges from 2.6 to 7.4%, and most cases of cholangitis are considered to be related to biliary strictures [10, 11, 25]. In contrast, in the present study, over 50% of patients who developed refractory cholangitis did not have biliary strictures. Cholangitis is known to occur even in the absence of intrahepatic biliary duct dilatation [26]. Some case reports on postoperative cholangitis without biliary strictures after PD [27] or liver transplantation [28] indicated that reflux of bile or intestinal juice into the biliary tree is responsible for cholangitis. Another case report suggested that non-obstructive afferent loop syndrome caused biliary stasis, leading to cholangitis [29].
Biliary strictures are generally considered to be manageable by radiologic or endoscopic dilatation. [25, 30] Early diagnosis and prompt treatment of biliary strictures are essential to prevent secondary biliary cirrhosis [31]. Dilatation interventions are often needed repeatedly. According to a previous study, an average of 7.5 interventional treatments for obstructive cholangitis was required [10]. In this study, long-term remission was achieved in all 10 patients with an average of 2 dilatation treatments. Prawdzik et al. demonstrated that surgical re-reconstruction of hepaticojejunostomy strictures could be safely performed by an experienced pancreatic surgeon with low morbidity, and aggressive re-operation of hepaticojejunostomy was associated with optimal long-term outcomes [7]. However, this may be over-optimistic because re-operation can be challenging, and minimally invasive treatment such as radiologic or endoscopic dilatation should be performed as an initial step.
For patients with biliary strictures, dilatation of the stricture is a curative treatment. On the other hand, in patients without biliary strictures, cholangitis was usually not very severe and usually resolved through pharmacotherapy, but recurred repeatedly. Such treatment was not sufficient to result in a cure because the potential cause was not identified. Pharmacotherapy, mainly antibiotics, may be an acceptable means of management as a desperate measure. We established a flowchart on the management of refractory cholangitis (Fig. 3).
In this study, 5 risk factors for refractory cholangitis were identified: benign disease, long operation time, elevated CRP, ALP, and the presence of pneumobilia after discharge. There were 14 patients with benign disease in the refractory cholangitis group, 9 of which (64.3%) had biliary strictures. On the other hand, only one patient (14.3%) with malignant disease had biliary stricture. Benign disease was a risk factor for refractory cholangitis because patients with benign disease had a non-dilated common bile duct. Long operation time was also a risk factor for refractory cholangitis. Because the total operation time was affected by case-specific difficulties, such difficulties may underlie the relationship between operation time and the risk of refractory cholangitis. Elevated CRP and ALP levels were also risk factors for refractory cholangitis, but these laboratory data may be influenced by several factors. Postoperative pneumobilia after PD is a common finding, being observed in 60–90% of PD patients [32,33,34]. This study identified pneumobilia after PD as a risk factor for refractory cholangitis as it was detected in 76 patients (67.3%) regardless of biliary obstruction (Table 3). Among the 21 patients with refractory cholangitis, 19 patients had pneumobilia. In contrast, only 2 of the 37 patients without pneumobilia developed refractory cholangitis. It is noteworthy that patients without pneumobilia after PD rarely developed refractory cholangitis.
Our study was limited by its retrospective design and relatively small sample size. We reviewed past studies about postoperative cholangitis after PD; however, such studies were few and the definition of cholangitis varied among such studies. Prospective controlled studies with an international standardized definition of postoperative cholangitis after PD are needed. We believe that our findings might be of value for the future management of postoperative cholangitis after PD.
Conclusions
Postoperative refractory cholangitis after PD usually developed within a year. Almost half of the patients had biliary strictures, and aggressive dilatation might be effective to achieve remission in these patients.
References
Cameron JL, He J (2015) Two thousand consecutive pancreaticoduodenectomies. J Am Coll Surg 220:530–536
Ahmad SA, Edwards MJ, Sutton JM et al (2012) Factors influencing readmission after pancreaticoduodenectomy: a multi-institutional study of 1302 patients. Ann Surg 256:529–537
Yeo CJ, Cameron JL, Sohn TA et al (1997) Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg 226:248–257 (discussion 257–260)
Amini N, Spolverato G, Kim Y et al (2015) Trends in hospital volume and failure to rescue for pancreatic surgery. J Gastrointest Surg 19:1581–1592
Kimura W, Miyata H, Gotoh M et al (2014) A pancreaticoduodenectomy risk model derived from 8575 cases from a national single-race population (Japanese) using a web-based data entry system: the 30-day and in-hospital mortality rates for pancreaticoduodenectomy. Ann Surg 259:773–780
Keck T, Wellner UF, Bahra M et al (2016) Pancreatogastrostomy versus pancreatojejunostomy for RECOnstruction after PANCreatoduodenectomy (RECOPANC, DRKS 00000767): perioperative and long-term results of a multicenter randomized controlled trial. Ann Surg 263:440–449
Prawdzik C, Belyaev O, Chromik AM et al (2015) Surgical revision of hepaticojejunostomy strictures after pancreatectomy. Langenbecks Arch Surg 400:67–75
Kang CM, Lee JH (2015) Pathophysiology after pancreaticoduodenectomy. World J Gastroenterol 21:5794–5804
Nomura R, Ishizaki Y, Suzuki K et al (2007) Development of hepatic steatosis after pancreatoduodenectomy. Am J Roentgenol 189:1484–1488
House MG, Cameron JL, Schulick RD et al (2006) Incidence and outcome of biliary strictures after pancreaticoduodenectomy. Ann Surg 243:571–576 (discussion 576–578)
Duconseil P, Turrini O, Ewald J et al (2014) Biliary complications after pancreaticoduodenectomy: skinny bile ducts are surgeons’ enemies. World J Surg 38:2946–2951
Yamaguchi K, Tanaka M, Chijiiwa K et al (1999) Early and late complications of pylorus-preserving pancreatoduodenectomy in Japan 1998. J Hepatobiliary Pancreat Surg 6:303–311
Janbain M, Leissinger CA, Kruse-Jarres R (2015) Acquired hemophilia A: emerging treatment options. J Blood Med 6:143–150
Wada K, Takada T, Kawarada Y et al (2007) Diagnostic criteria and severity assessment of acute cholangitis: Tokyo guidelines. J Hepatobiliary Pancreat Surg 14:52–58
Kiriyama S, Takada T, Strasberg SM et al (2012) New diagnostic criteria and severity assessment of acute cholangitis in revised Tokyo guidelines. J Hepatobiliary Pancreat Sci 19:548–556
Kimura W (2008) Strategies for the treatment of invasive ductal carcinoma of the pancreas and how to achieve zero mortality for pancreaticoduodenectomy. J Hepatobiliary Pancreat Surg 15:270–277
Friess H, Ho CK, Kleef J et al (2007) Pancreaticoduodenectomy, distal pancreatectomy, segmental pancreatectomy, total pancreatectomy, and transduodenal resection of the papilla of Vater. In: Surgery of the liver, biliary tract, and pancreas. Saunders, Philadelphia, pp 877–903
Fujii T, Sugimoto H, Yamada S et al (2014) Modified Blumgart anastomosis for pancreaticojejunostomy: technical improvement in matched historical control study. J Gastrointest Surg 18:1108–1115
Bassi C, Dervenis C, Butturini G et al (2005) Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 138:8–13
Wente MN, Bassi C, Dervenis C et al (2007) Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 142:761–768
Wente MN, Bassi C, Dervenis C et al (2007) Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 142:20–25
Koch M, Garden OJ, Padbury R et al (2011) Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery 149:680–688
Westerkamp AC, Korkmaz KS, Bottema JT et al (2015) Elderly donor liver grafts are not associated with a higher incidence of biliary complications after liver transplantation: results of a national multicenter study. Clin Transplant 29:636–643
Higuchi R, Takada T, Strasberg SM et al (2013) TG13 miscellaneous etiology of cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci 20:97–105
Reid-Lombardo KM, Ramos-De la Medina A, Thomsen K et al (2007) Long-term anastomotic complications after pancreaticoduodenectomy for benign diseases. J Gastrointest Surg 11:1704–1711
Chuang JH, Lee SY, Chen WJ et al (2001) Changes in bacterial concentration in the liver correlate with that in the hepaticojejunostomy after bile duct reconstruction: implication in the pathogenesis of postoperative cholangitis. World J Surg 25:1512–1518
Tsalis K, Antoniou N, Koukouritaki Z et al (2014) Successful treatment of recurrent cholangitis by constructing a hepaticojejunostomy with long Roux-en-Y limb in a long-term surviving patient after a Whipple procedure for pancreatic adenocarcinoma. Am J Case Rep 15:348–351
Vrochides D, Fischer SA, Soares G et al (2007) Successful treatment of recurrent cholangitis complicating liver transplantation by Roux-en-Y limb lengthening. Transpl Infect Dis 9:327–331
Sanada Y, Yamada N, Taguchi M et al (2014) Recurrent cholangitis by biliary stasis due to non-obstructive afferent loop syndrome after pylorus-preserving pancreatoduodenectomy: report of a case. Int Surg 99:426–431
Bonnel DH, Fingerhut AL (2012) Percutaneous transhepatic balloon dilatation of benign bilioenteric strictures: long-term results in 110 patients. Am J Surg 203:675–683
Ammori BJ, Joseph S, Attia M et al (2000) Biliary strictures complicating pancreaticoduodenectomy. Int J Pancreatol 28:15–21 (discussion 21–22)
Sherman SC, Tran H (2006) Pneumobilia: benign or life-threatening. J Emerg Med 30:147–153
Mortelé KJ, Lemmerling M, de Hemptinne B et al (2000) Postoperative findings following the Whipple procedure: determination of prevalence and morphologic abdominal CT features. Eur Radiol 10:123–128
Gutknecht DR (2001) Cholangitis and pneumobilia after a Whipple procedure. Am J Emerg Med 19:87–88
Acknowledgements
We would like to thank the radiologists and gastroenterologists at our hospital who greatly contributed to the clinical diagnosis and treatment of cholangitis in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Meeting presentation information: Presented as a poster presentation at the Society for Surgery of the Alimentary Tract poster session (Poster ID: Tu1504) in Digestive Disease Week 2016, May 24, 2016, San Diego, California, and at the 50th Annual Pancreas Club poster session (Poster ID: P073), May 20, 2016, San Diego, California.
Rights and permissions
About this article
Cite this article
Ueda, H., Ban, D., Kudo, A. et al. Refractory Long-Term Cholangitis After Pancreaticoduodenectomy: A Retrospective Study. World J Surg 41, 1882–1889 (2017). https://doi.org/10.1007/s00268-017-3912-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-017-3912-z