Abstract
Background
Enhanced recovery after surgery (ERAS) programs have been shown to ease the postoperative recovery and improve clinical outcomes for various surgery types. ERAS cost-effectiveness was demonstrated for colorectal surgery but not for liver surgery. The present study aim was to analyze the implementation costs and benefits of a specific ERAS program in liver surgery.
Methods
A dedicated ERAS protocol for liver surgery was implemented in our department in July 2013. The subsequent year all consecutive patients undergoing liver surgery were treated according to this protocol (ERAS group). They were compared in terms of real in-hospital costs with a patient series before ERAS implementation (pre-ERAS group). Mean costs per patient were compared with a bootstrap T test. A cost-minimization analysis was performed.
Results
Seventy-four ERAS patients were compared with 100 pre-ERAS patients. There were no significant pre- and intraoperative differences between the two groups, except for the laparoscopy number (n = 18 ERAS, n = 9 pre-ERAS, p = 0.010). Overall postoperative complications were observed in 36 (49 %) and 64 patients (64 %) in the ERAS and pre-ERAS groups, respectively (p = 0.046). The median length of stay was significantly shorter for the ERAS group (8 vs. 10 days, p = 0.006). The total mean costs per patient were €38,726 and €42,356 for ERAS and pre-ERAS (p = 0.467). The cost-minimization analysis showed a total mean cost reduction of €3080 per patient after ERAS implementation.
Conclusions
ERAS implementation for liver surgery induced a non-significant decrease in cost compared to standard care. Significant decreased complication rate and hospital stay were observed in the ERAS group.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Enhanced recovery after surgery (ERAS) programs are standardized multimodal perioperative care aiming to reduce the patient’s response to surgical stress and to decrease the postoperative complication risk [1]. Fewer postoperative complications and improved recovery lead to a shorter length of hospital stay (LoS). ERAS has been shown to be beneficial on a clinical and economic level in colorectal surgery [2–4]. Therefore, ERAS programs have been extended to other types of surgery, including liver surgery [5–10], but few studies compared an ERAS pathway for liver surgery to a conventional perioperative management [11–14]. The preliminary results have shown that ERAS for liver surgery appears to be safe and feasible [12–17]. Moreover, some studies showed a diminution of postoperative complications or LoS [18–20].
Formal guidelines for ERAS in liver surgery have not been published yet. However, general recommendations for ERAS in liver surgery based on ERAS protocol in pancreas surgery [21] encompass the use of preoperative counseling, carbohydrate beverages, reduced preoperative fasting, optimized fluid balance, avoidance of premedication, standardized postoperative analgesia, early postoperative nutrition, and early postoperative mobilization.
While implementing a new clinical perioperative pathway should first and foremost result in clinical benefits for the patients, it should also be preferentially cost-beneficial in order to convince the financial hospital authorities to invest in such a project and to contribute to reduce the health care costs.
The study aim was to compare the complete real in-hospital costs for liver resections before and after systematic implementation of an ERAS pathway for liver surgery in an ERAS-dedicated tertiary center.
Materials and methods
ERAS protocol and patient groups
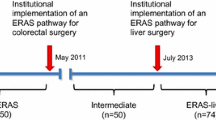
ERAS was first implemented for colorectal surgery in our department in May 2011. The members of the multidisciplinary team had received beforehand a formal training provided by the ERAS® Society. ERAS protocol for liver surgery was implemented in the department of Visceral Surgery of the University Hospital of Lausanne (CHUV, Switzerland) in July 2013. This protocol was developed by the local ERAS team based on previous ERAS protocols for pancreas [21] and colorectal [22] surgery. This liver protocol encompasses a list of diverse pre-, intra-, and postoperative items that are summarized in detail in Table 1.
From July 2013 to July 2014, all consecutive liver resections were included into ERAS. Major hepatectomy was defined as resection of ≥3 Couinaud segments, and minor hepatectomy as resection of one to two liver segments. Wedge resections were defined as non-anatomical partial resections of liver segments.
The ERAS group (from July 2013 to July 2014) was compared to a pre-ERAS group that included a cohort of 100 consecutive patients who underwent liver surgery before the implementation of ERAS for liver surgery. No power analysis was performed because it was estimated a priori that selecting a cohort of 70–80 consecutive ERAS patients would permit to draw a firm statistically based conclusion.
The study was retrospective. However, all data were prospectively recorded in our liver database for both periods, prior and after ERAS implementation. Data collection was performed by a dedicated and fully trained ERAS nurse. The study was approved by the local ethics committee (protocol number 362/14) and was registered online on Research Registry (UIN: researchregistry443).
Perioperative parameters
Operative time was calculated from incision to skin closure, whereas anesthesia time was calculated from the patient entry in the operating room (OR) until the patient was awake. Postoperative complications were graded according to the validated Clavien classification [23] and the comprehensive complication index (CCI) [24]. Grade I–II were defined as minor complications and grade III–IV as major complications [23]. Postoperative death during the hospital stay or during the 30 postoperative days was defined as grade V [23]. Formal ERAS-validated discharge criteria were as follows: control of pain with oral analgesia only, no intravenous fluids, independent mobilization, and sufficient oral intake (at least 2/3 of a normal meal). LoS was defined from operation day until day of discharge. Data about readiness for discharge were not collected. Although readiness for discharge is more realistic than LoS, this concept may be more subjective (based on doctors’ appreciations) than the actual hospital LoS. That is why LoS was calculated based on objective figures. Readmission rate was defined as the number of patients needing a rehospitalization within 60 days of surgery. Overall compliance was calculated as the number of fulfilled pre-, intra-, and postoperative items divided by the total number of predefined enhanced recovery measures (expressed in %). For the pre-ERAS group, it was assessed if some ERAS items were already applied, and overall compliance was then calculated.
Analysis of the costs
A comprehensive analysis of all real costs was performed for every patient according to a method previously used by our group [3]. Briefly, all pertinent intraoperative costs and pre/postoperative costs were considered. The intraoperative costs included the disposable material used during the operation and the anesthesia as well as OR costs. The anesthesia costs integrated the anesthesiologist and anesthesia nurse costs (based on the duration of the anesthesia in minutes) as well as the materials and medications used for the anesthesia. The OR costs were based on the OR occupation in minutes. The pre- and postoperative costs included the following items: intensive care unit (ICU)/intermediate care (IC), medical care, nursing care, physiotherapy, medication, blood test, laboratory, radiology, pathology, housing, administration, and other costs. The medical care costs included all costs related to the doctors’ clinical activities, except for the anesthesiologist costs accounted in the anesthesia and OR costs. The costs of nursing care represented the costs of the ward and did not include the ICU and IC costs. They were based on the Project of Research in Nursing (PRN) which determines the prospective nursing time needed per patient based on a validated 249-item list [25]. The housing costs were calculated per day of hospitalization. The administrative costs were counted per admission. Finally, the other costs covered the social work division, the priest, and the occupational therapy. Detailed data were furnished by the account department of our hospital and represented the real costs of each patient case.
All costs were primarily in Swiss Francs (CHF). The used exchange rate to euros (€) was the one current on March 8, 2015: CHF1 = €0.94. No correction for the general price difference between the two periods (ERAS and pre-ERAS) was made.
Cost-minimization analysis and sensitivity analysis
A cost-minimization analysis was performed, i.e., hospital savings per patient were calculated. This present analysis was made by the subtraction of the standard care costs per patient to the ERAS-specific costs per patient and the ERAS costs per patient. The ERAS-specific costs included the salary of the ERAS-dedicated nurse (fixed costs), the costs of the quarterly ERAS liver meetings (fixed costs), the ERAS database (variable costs, depending on the patient number), the patient carbohydrates drinks (variable costs, depending on the patient number), and the patient logbook costs (variable costs, depending on the patient number).
A sensitivity analysis was undertaken as well. As fixed costs are independent from the patient number and are allocated on an individual basis, large variations due to the number of patients can appear. We therefore calculated the cost-minimization by varying the patient number by ±50 % (i.e., calculating the ERAS-specific costs for 37 and 111 patients).
Subgroup analysis
A subgroup analysis was performed for all hepatic resections that were performed by laparotomy. Mean total costs were calculated and compared using a bootstrap T test.
Statistical analysis
A Mann–Whitney U test or a T test was used to compare continuous variables depending on the distribution type and the homogeneity of the variances. Categorical (discrete) variables were compared using a Fisher’s exact test or a Chi-square test. The arithmetic means were used because they represent informative and explicit figures from a pharmaco-economic standpoint. Moreover, a resampling was performed for the cost analysis by using the bootstrap method because of its simplicity, its property to derive confidence intervals from a complex sample distribution, and its robustness of estimation. A bootstrap T test was used to compare the different costs. A p value <0.05 was considered statistically significant. Statistical analyses were performed using SPSS®19 (IBM, Armonk, New York, USA) and GraphPad Prism©5.
Results
Patient characteristics and surgical details
A total of 174 patients were enrolled for further analysis: 74 in the ERAS group and 100 in the pre-ERAS group. Demographic, preoperative, and surgical data were comparable in the ERAS and pre-ERAS groups, except for laparoscopy that was more frequently performed in the ERAS group (n = 18 vs. n = 9, p = 0.010). Table 2 resumes the patient characteristics and the surgery type performed.
Compliance and perioperative outcomes
In terms of compliance to the ERAS protocol, the overall rate including all pre-, intra-, and postoperative ERAS items was 73.8 % in the ERAS group compared to 48.7 % in the pre-ERAS group (p < 0.001). The readmission rate 60 days after the operation was not significantly different between the two groups (6/74 = 8 % and 7/100 = 7 %, p = 0.780).
The perioperative data are summarized in Table 3. There was no difference in the operative time and the anesthesia time between the ERAS and pre-ERAS groups. The overall complication rate was significantly lower in the ERAS group compared to the pre-ERAS group (36/74 = 49 % vs. 64/100 = 64 %, p = 0.046). If one subdivides the complications into minor (I–II) and major (III–IV), there were no differences between the ERAS and pre-ERAS groups. Median CCI was lower for the ERAS group (8.7 vs. 20.9, p = 0.044). The median LoS was significantly shorter for the ERAS group (8 vs. 10 days, p = 0.006). The median ICU stays were 0 and 1 day for the ERAS and pre-ERAS groups (p < 0.001), while the median IC stays were 2 and 3 days (p = 0.002), respectively.
Analysis of the costs
The mean (SD) total costs/patient were €38,726 (31,608) for the ERAS group and €42,356 (26,898) for the pre-ERAS group (p = 0.467). The mean intraoperative costs/patient for the ERAS and pre-ERAS groups were €10,793 (6228) and €9981 (4440), respectively (p = 0.236). The mean pre-/postoperative costs/patient were €27,933 (27,635) for the ERAS group and €32,375 (25,224) for the pre-ERAS group (p = 0.271). The pre-, intra-, and postoperative costs are summarized in detail in Table 4 and Fig. 1. All mean costs/patient were lower for the ERAS group except for the anesthesia and OR, the nursing care, the housing, the administration, and the social service, occupational therapy, and priest costs.
Cost-minimization analysis and sensitivity analysis
The difference of mean total costs/patient between the pre-ERAS and ERAS groups was €3630 (−9 %), meaning that the total mean costs of ERAS/patient were €3630 less expensive than the ones of the pre-ERAS group.
In the ERAS-specific costs, fixed ERAS costs included the crude salary of the ERAS-dedicated nurse (€81,845 per year) and the costs of the ERAS liver meetings (€50/meeting for the used material and the preparation time, at least four meetings/year). As the ERAS-dedicated nurse was also responsible for ERAS colorectal and ERAS pancreas during the same period of time, we divided her salary by three. Fixed ERAS costs/patient were therefore: 27,282/74 + 200/74 = €371. The variable ERAS costs were the ERAS database (€100/patient), the patient carbohydrate drinks (€75/patient), and the patient logbooks (€4/patient). ERAS-specific costs were thus calculated to be €550/patient.
The final total gain/patient for the ERAS group was €3080 (−7 %).
If we vary the number of patients by ±50 %, the ERAS-specific costs/patient would be €922 for 37 patients and €427 for 111 patients, leading to a final total gain for ERAS/patient of €2708 (−6 %) and €3203 (−8 %), respectively.
Subgroup analysis
Regarding the operations performed by laparotomy, there were 55 patients in the ERAS group and 92 patients in the pre-ERAS group. Both groups showed similar demographics and surgical details. The total mean costs/patient was €42,234 (39,526) for the ERAS group and €45,584 (35,985) for the pre-ERAS group (difference: €3350, p = 0.657).
Discussion
The present study assessing the real costs of implementation of an ERAS protocol for liver surgery demonstrated in our cohort a non-significant decrease in costs compared to standard management but a significant decrease in complications and LoS.
The main savings engendered by the implementation of ERAS for liver surgery were found in the ICU/IC costs (€2578/patient), but were not statistically significant (p = 0.174). The majority of patients included in the ERAS protocol did not need to stay in the ICU, while the median length of ICU stay was 1 day for the pre-ERAS group (p < 0.001). Moreover, the reduction of ICU stay for the ERAS group was also coupled to a shorter IC stay compared to the pre-ERAS group (2 vs. 3 days, p = 0.002). This cannot be explained by a difference of operation complexity (major vs. minor hepatectomy) as the ERAS and pre-ERAS groups were similar in this regard (p = 0.643) and the operation durations were comparable (p = 0.188). With ERAS liver implementation, patients were routinely scheduled for IC unit if needed, or for the standard ward. Only in rare case of extended major hepatectomy, patients were postoperatively transferred to the ICU. The fact that IC unit stay was also shorter for the ERAS group suggests an enhanced recovery in the early postoperative days and a lower rate of patients necessitating continuous monitoring. This could be related to the standardized anesthesia protocol that emphasized the postoperative analgesia, the postoperative nausea and vomiting prophylaxis, the fluid intake, and the early diet, and/or to the standardized care maps guiding the postoperative phase [26, 27].
The second main absolute gain in the ERAS group but not statistically significant was related to the medical care costs (€1052/patient). This can be linked, if there is a gain as it was not significant, to the reduced overall complication rate and LoS observed in the ERAS group. A postoperative complication results in increased medical resources (consultation, non-surgical procedures, etc.), therefore increasing the medical care costs [28, 29]. ERAS also had a significant cost-benefit effect on the radiology and a non-statistically significant cost-benefit effect on the medication. The radiology costs represented the main statistically significant savings of the ERAS group with a gain of €558/patient (p = 0.021). In the ERAS group, fewer medications were used and fewer radiological exams were performed postoperatively. These can be an effect of the implementation of ERAS, can be due to the diminution of postoperative complications, or can just be due to the standardization of the postoperative management with the clinical care maps [27], or a mix of the three previous explanations. Costs are frequently linked to LoS, and LoS differ widely from country to country. The most important factor for one country is the difference between the LoS before and after ERAS implementation rather than the absolute LoS. The same is true for ICU and IC stays that depend on local habits and infrastructures; the important point being the difference in ICU and IC needs before and after ERAS implementation more than the absolute numbers.
Conversely, the ERAS group had significantly higher anesthesia/OR costs and not significantly higher nursing care costs. As the anesthesia and OR times were not different between the two groups, the difference of costs mainly lies in the material and drugs used during anesthesia. The standardized anesthesia protocol and the routine prophylactic use of postoperative nausea and vomiting prevention by droperidol, ondansetron, and betamethasone only explain a small part of this difference, as the prophylaxis costs are low. The higher number of laparoscopies in the ERAS group had no impact on the material and drugs used during anesthesia. Of note, the number of thoracic epidurals was similar in both groups (54/74 ERAS group vs. 79/100 pre-ERAS group, p = 1). No clear explanation was therefore found for this increase in anesthesia and OR costs. Regarding the nursing care costs, as the ICU and IC median stays were shorter, the patients with higher PRN number (i.e., representing the case complexity in terms of nursing actions) [25] were transferred earlier to the ward, therefore increasing this part of the costs at the beginning of their hospital stay.
Length of hospital stay was found to be shorter in the ERAS group. There was nevertheless no repercussion on the mean housing costs/patient. No difference of mean housing costs was indeed found between the ERAS and pre-ERAS groups (p = 0.058). This is explained by the constant annual increase (inflation) of the basal rate of hospital housing costs throughout the years. It is not clear why a diminution of complications and a shorter LoS did not translate into significant overall cost difference. One explanation could be related to the large found confidence intervals inherent to the bootstrap method and related to the p value. It is nevertheless interesting to notice that implementation of ERAS had positive outcomes for the patients without increasing the costs, meaning that ERAS was cost-effective. This also justifies the investment costs necessary to implement ERAS.
ERAS for liver surgery was previously shown to be feasible and safe [12–15]. Two systematic reviews showed a decrease in complications and/or a reduction of LoS without an increase of the readmission rate, corroborating the results of this present study [18, 19]. A study by Dunne et al. including hepatectomies for colorectal metastasis found that as ERAS experience increases with time, a progressive diminution of hospitalization time and critical care admission was noticed [30]. However, reduced LoS may be associated with higher rate of readmissions as reported by Connor et al. [11]. It has to be emphasized that the present study showed a significant shorter LoS after ERAS implementation, but the readmission rate did not increase and remained low. Hospital discharge was based on formal discharge criteria.
As shown by previous studies, implementation of ERAS protocols in gastrointestinal surgery needs initial investments, but is then associated with an important gain per patient for both financial and clinical aspects (significant diminution of complications, reduction of LoS, quicker recovery) [2, 3, 31–33]. Once the implementation phase is done, financial gains appear and continue over time, giving considerable savings proportionally to the increasing number of patients included in the protocol. Regarding the real costs of the implementation of an ERAS pathway for liver surgery, data are for the moment non-existent. A simulation study from Faujour et al. was recently published and estimated that after ERAS implementation in different specialties, an overall gain of €180/hospital day for all the surgical units could be obtained [34]. In this study, ERAS was implemented in five French units of digestive (including liver surgery), orthopedic, and urology surgeries. Their outcomes were based on estimations and not on real costs as in the present study. In our department, ERAS was already implemented in colorectal surgery since 2011, so costs of education and training of the team were not included in this analysis.
Comparing the overall cost data of this study to the results published for colorectal surgery [3], the absolute cost difference between ERAS and pre-ERAS is more important in liver surgery (€3080 vs. €1651). This is partly explained by the fact that the overall costs for liver surgery are higher than for colorectal surgery. The observed not significant overall cost difference between ERAS and pre-ERAS in the present study can be explained by the fact that even though there is an important absolute difference (i.e., €3080) between the two groups, the relative difference is rather small (−7 %). Nevertheless, the present study suggests benefits of ERAS implementation in liver surgery.
The present study has several limitations that must be addressed. First, the higher number of laparoscopies in the ERAS group may in part explain the results. Laparoscopy as single measure has been shown to improve and fasten recovery [35, 36]. Therefore, the higher percentage of laparoscopic cases in the ERAS group entails a bias, as we do not know exactly to which extent the benefits are due to ERAS, laparoscopy, standardization, or all together. To minimize this bias, a subgroup analysis was performed with the patients operated by laparotomy. The difference of total mean costs/patient between pre-ERAS and ERAS (€3350) was not different from the total mean costs calculated in the entire cohort (€3620, laparotomy and laparoscopy patients). These findings support the fact that laparoscopy did not play a major role in the cost benefits observed in the ERAS group. Second, the ERAS and pre-ERAS groups were retrospectively analyzed inducing all possible limitations of a retrospective study (e.g., underreporting of complications, missing data). However, the data have been prospectively recorded in our liver database for several years, and the same database was used for both periods. Moreover, the validated Clavien classification of complications and the CCI were systematically used and provided a recognized objective evaluation of complications for both periods. Finally, only costs linked to the primary hospitalization were calculated (in-hospital costs), the costs for postoperative rehabilitation and recovery time before returning to work were not taken into account in the current analysis. However, despite the lack of actual data on rehabilitation after ERAS, it can be assumed that outpatient recovery may also become easier and faster in ERAS patients [37]. To support this statement discharge was based on formal pre-established criteria, and readmission rates were similar.
ERAS-skeptics may argue that it is difficult to differentiate between the positive benefits of the ERAS program itself and the systematization of care induced by the ERAS care maps. They are right, there is nothing magical in ERAS. However, care maps belong to ERAS programs with systematization of care, and the final goal achieved is of importance: a significant decrease in complication rate. Whether it comes from the pathophysiology of ERAS, from the systematization, or from both matters little but this deserves further investigation beyond the present study aim. Of note, the ERAS-specific costs of this present analysis were half of the ones that were published for implementation of ERAS in colorectal surgery [3] because the costs of the courses to educate the team to the ERAS concept were not necessary and therefore not included. Finally, one can challenge if Swiss results may be extrapolated to other countries. In fact, the importance of the present results lies in the cost, complication rate, and LoS differences between pre- and after ERAS implementation more than in the absolute numbers. For this reason, it can be suggested that the present outcomes may also be observed in other countries.
In conclusion, implementation of ERAS for liver surgery showed a non-significant decrease in costs in our institution. It also led to a significant reduction of the overall complication rate and LoS.
References
Lassen K, Soop M, Nygren J et al (2009) Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg 144:961–969
Greco M, Capretti G, Beretta L et al (2014) Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg 38:1531–1541. doi:10.1007/s00268-013-2416-8
Roulin D, Donadini A, Gander S et al (2013) Cost-effectiveness of the implementation of an enhanced recovery protocol for colorectal surgery. Br J Surg 100:1108–1114
Lemanu DP, Singh PP, Stowers MDJ et al (2014) A systematic review to assess cost effectiveness of enhanced recovery after surgery programmes in colorectal surgery. Colorectal Dis 16:338–346
Sahoo MR, Gowda MS, Kumar AT (2014) Early rehabilitation after surgery program versus conventional care during perioperative period in patients undergoing laparoscopic assisted total gastrectomy. J Minim Access Surg 10:132–138
Kagedan DJ, Ahmed M, Devitt KS et al (2015) Enhanced recovery after pancreatic surgery: a systematic review of the evidence. HPB 17:11–16
Findlay JM, Gillies RS, Millo J et al (2014) Enhanced recovery for esophagectomy: a systematic review and evidence-based guidelines. Ann Surg 259:413–431
Arsalani-Zadeh R, ElFadl D, Yassin N et al (2011) Evidence-based review of enhancing postoperative recovery after breast surgery. Br J Surg 98:181–196
Coolsen MME, van Dam RM, van der Wilt AA et al (2013) Systematic review and meta-analysis of enhanced recovery after pancreatic surgery with particular emphasis on pancreaticoduodenectomies. World J Surg 37:1909–1918. doi:10.1007/s00268-013-2044-3
Findlay JM, Tustian E, Millo J et al (2015) The effect of formalizing enhanced recovery after esophagectomy with a protocol. Dis Esophagus 28:567–573
Connor S, Cross A, Sakowska M et al (2013) Effects of introducing an enhanced recovery after surgery programme for patients undergoing open hepatic resection. HPB 15:294–301
van Dam RM, Hendry PO, Coolsen MME et al (2008) Initial experience with a multimodal enhanced recovery programme in patients undergoing liver resection. Br J Surg 95:969–975
Jones C, Kelliher L, Dickinson M et al (2013) Randomized clinical trial on enhanced recovery versus standard care following open liver resection. Br J Surg 100:1015–1024
Stoot JH, van Dam RM, Busch OR et al (2009) The effect of a multimodal fast-track programme on outcomes in laparoscopic liver surgery: a multicentre pilot study. HPB 11:140–144
Hall TC, Dennison AR, Bilku DK et al (2012) Enhanced recovery programmes in hepatobiliary and pancreatic surgery: a systematic review. Ann R Coll Surg Engl 94:318–326
Dasari BVM, Rahman R, Khan S et al (2015) Safety and feasibility of an enhanced recovery pathway after a liver resection: prospective cohort study. HPB 17:700–706
Schultz NA, Larsen PN, Klarskov B et al (2013) Evaluation of a fast-track programme for patients undergoing liver resection. Br J Surg 100:138–143
Hughes MJ, McNally S, Wigmore SJ (2014) Enhanced recovery following liver surgery: a systematic review and meta-analysis. HPB 16:699–706
Coolsen MME, Wong-Lun-Hing EM, van Dam RM et al (2013) A systematic review of outcomes in patients undergoing liver surgery in an enhanced recovery after surgery pathways. HPB 15:245–251
Lei Q, Wang X, Tan S et al (2014) Fast-track programs versus traditional care in hepatectomy: a meta-analysis of randomized controlled trials. Dig Surg 31:392–399
Lassen K, Coolsen MME, Slim K et al (2013) Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. World J Surg 37:240–258. doi:10.1007/s00268-012-1771-1
Gustafsson UO, Scott MJ, Schwenk W et al (2012) Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr 31:783–800
Dindo D, Demartines N, Clavien P-A (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Slankamenac K, Nederlof N, Pessaux P et al (2014) The comprehensive complication index: a novel and more sensitive endpoint for assessing outcome and reducing sample size in randomized controlled trials. Ann Surg 260:757–762
Chagnon M, Audette LM, Lebrum L et al (1978) A patient classification system by level of nursing care requirements. Nurs Res 27:107–112
Giglio MT, Marucci M, Testini M et al (2009) Goal-directed haemodynamic therapy and gastrointestinal complications in major surgery: a meta-analysis of randomized controlled trials. Br J Anaesth 103:637–646
Müller MK, Dedes KJ, Dindo D et al (2009) Impact of clinical pathways in surgery. Langenbecks Arch Surg 394:31–39
Turrentine FE, Denlinger CE, Simpson VB et al (2015) Morbidity, mortality, cost, and survival estimates of gastrointestinal anastomotic leaks. J Am Coll Surg 220:195–206
Dimick JB, Chen SL, Taheri PA et al (2004) Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg 199:531–537
Dunne DFJ, Yip VS, Jones RP et al (2014) Enhanced recovery in the resection of colorectal liver metastases. J Surg Oncol 110:197–202
Lee L, Li C, Robert N et al (2013) Economic impact of an enhanced recovery pathway for oesophagectomy. Br J Surg 100:1326–1334
Stowers MDJ, Lemanu DP, Hill AG (2014) Health economics in Enhanced Recovery After Surgery programs. Can J Anaesth 62:219–230
Richardson J, Di Fabio F, Clarke H et al (2015) Implementation of enhanced recovery programme for laparoscopic distal pancreatectomy: feasibility, safety and cost analysis. Pancreatology 15:185–190
Faujour V, Slim K, Corond P (2015) The future, in France, of enhanced recovery after surgery seen from the economical perspective. Presse Med 44:e23–e31
Xiong J-J, Altaf K, Javed MA et al (2012) Meta-analysis of laparoscopic vs open liver resection for hepatocellular carcinoma. World J Gastroenterol 18:6657–6668
Cheung TT, Poon RTP, Yuen WK et al (2013) Long-term survival analysis of pure laparoscopic versus open hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a single-center experience. Ann Surg 257:506–511
McLeod RS, Aarts M-A, Chung F et al (2015) Development of an Enhanced Recovery After Surgery Guideline and Implementation Strategy Based on the Knowledge-to-action Cycle. Ann Surg 262:1016–1025
Acknowledgments
The authors would like to thank all the ERAS team of our institution for the daily clinical and research work. A particular thank is addressed to Valérie Addor our ERAS-dedicated nurse who gathered all patient data and to Ghada Jarrar who helped in the completion of the ERAS liver database. Mr. Nicolas Larqué of the accounting department is also acknowledged for providing us with all the real costs and financial patient data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Gaëtan-Romain Joliat, Ismaïl Labgaa, Martin Hübner, Catherine Blanc, Anne-Claude Griesser, Markus Schäfer, and Nicolas Demartines have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Joliat, GR., Labgaa, I., Hübner, M. et al. Cost–Benefit Analysis of the Implementation of an Enhanced Recovery Program in Liver Surgery. World J Surg 40, 2441–2450 (2016). https://doi.org/10.1007/s00268-016-3582-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-016-3582-2