Abstract
Background
Preoperative N staging is essential for the best treatment planning in patients with gastric carcinoma. The aim of this study was to evaluate the accuracy of preoperative N staging using contrast-enhanced multi-detector row computed tomography (CE-MDCT) in patients with resectable cT2-4 gastric carcinoma.
Methods
A total of 218 patients who underwent a gastrectomy with D2 lymphadenectomy for previously untreated cT2-4 primary gastric carcinoma were studied. Preoperative N staging was performed according to the 7th (UICC) TNM Staging System using pre-specified criteria on a 64-channel CE-MDCT and was compared with postoperative pathologic N staging.
Results
In all 218 patients, a distal or total gastrectomy was performed. The overall accuracy of the preoperative N staging was 46.3 % (101/218), with the proportion of over- and under-staging being 26.6 % (58/218) and 27.1 % (59/218), respectively. The sensitivity, specificity, and accuracy for lymph node metastasis (≥pN1) were 79.1 % (106/134), 50.0 % (42/84), and 67.9 % (148/218), respectively. The sensitivity, specificity, and accuracy for multiple lymph node metastases (≥pN2) were 80.2 % (73/91), 68.5 % (87/127), and 73.4 % (160/218), respectively. Multivariate analyses showed that macroscopic type 2 and ≥6 cm-sized tumors were associated with preoperative over-N staging, while macroscopic type 1/3 tumors were associated with under-N staging.
Conclusion
Preoperative N staging with pinpoint accuracy is difficult. However, CE-MDCT offers a reasonably high sensitivity and specificity for ≥pN2 and may be useful for selecting candidates for neoadjuvant therapies. The macroscopic type and size of the primary tumor may affect the accuracy of preoperative N staging.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gastric carcinoma is one of the leading causes of cancer death worldwide [1]. Early-stage tumors can be cured using less invasive treatments, including endoscopic resection [2, 3] and function-preserving gastrectomy [4, 5]. On the other hand, the outcomes of patients with locally advanced tumors remain unsatisfactory even after radical operations [6]. Currently, the most promising strategies to improve the outcomes of locally advanced gastric carcinomas include neoadjuvant and perioperative adjuvant therapies [7–9]. When considering preoperative therapies, however, clinical staging of the disease with the greatest possible accuracy, as well as an understanding of the utility and limitations of each staging modality, are essential.
The T and N stages are the most reliable prognostic indicators for patients with resectable gastric carcinoma [10], and preoperative staging has been shown to have a potential prognostic value [11]. At present, endoscopic ultrasound and computed tomography (CT) are widely used for preoperative staging. Generally speaking, N staging, compared with T staging, is still unreliable and leaves much room for improvement [12, 13]. In addition, preoperative N staging according to the latest 7th International Union Against Cancer (UICC) TNM Staging System [14] has yet to be fully evaluated [15].
Multi-detector row CT (MDCT) allows rapid scanning of a large longitudinal volume and provides objective images with prominent spatial resolution. Because of its ability to visualize the vascular anatomy precisely, MDCT is useful not only for staging, but also for operative planning [16–18]. Thus, MDCT is now one of the most important preoperative examinations performed in patients with gastric carcinoma.
The aim of this study was to evaluate the accuracy of preoperative N staging using contrast-enhanced MDCT (CE-MDCT) in patients with cT2-4 (invading the muscularis propria or deeper) gastric carcinoma. The diagnostic accuracy for multiple (three or more) lymph node metastases (≥pN2), which may be a good target for neoadjuvant therapies, was also assessed. The tumor characteristics associated with preoperative over- and under-N staging were explored.
Patients and methods
Patients
Between May 2007 and August 2009, a total of 929 patients with histologically proven gastric carcinoma underwent gastrectomy at our institution. Among these patients, the prospectively collected data of 218 patients with previously untreated, apparently resectable cT2-4 tumors who underwent CE-MDCT followed by a gastrectomy with D2 lymphadenectomy were studied. The remaining 711 patients who did not undergo CE-MDCT, who underwent a gastrectomy with <D2 lymphadenectomy, who had cT1 tumors, who had received neoadjuvant therapies, who had remnant gastric carcinoma, who had apparent distant metastasis, or who concurrently had active malignant or inflammatory diseases were excluded. Among all the enrolled patients, a preoperative diagnosis of cT2-4 was made based on conventional endoscopy findings with or without endoscopic ultrasound. This study was approved by the institutional review board (Ethics Committee National Cancer Center 2014-642).
Description of clinicopathologic data and operative procedures
The T and N stages were recorded according to the International Union Against Cancer (UICC) TNM Staging System [14]. The N stage was determined by the number of metastatic regional lymph nodes as follows: no metastasis (N0); one or two metastatic nodes (N1); three to six metastatic nodes (N2); and seven or more metastatic nodes (N3). The histologic type of tumor, macroscopic type of tumor, and lymph node station were recorded according to the Japanese Classification of Gastric Carcinoma [19]. Papillary adenocarcinoma and well or moderately differentiated tubular adenocarcinoma were described as differentiated-type carcinoma, while poorly differentiated adenocarcinoma, signet-ring cell carcinoma, and mucinous adenocarcinoma were classified as undifferentiated-type carcinoma.
Operative procedures were recorded according to the Japanese gastric cancer treatment guidelines [20]. Regional lymph nodes were defined as stations 1, 2, 3a, 3b, 4sa, 4sb, 4d, 5, 6, 7, 8a, 9, 10, 11p, 11d, and 12a for total gastrectomy and as stations 1, 3a, 3b, 4sb, 4d, 5, 6, 7, 8a, 9, 11p, and 12a for distal gastrectomy. All of these lymph nodes were dissected in a D2 procedure. The lymph nodes were collected from the resected specimen immediately after the surgery, divided according to station, and counted by the surgeons. The primary tumor and resected lymph nodes were then examined pathologically.
Protocol for CE-MDCT
All the patients provided written informed consent for CE-MDCT and were instructed to fast for at least 4 h before the examination. Only patients with normal renal function and those with no history of bronchial asthma or allergy to iodine were allowed to undergo the examination. The CE-MDCT examination was performed using a 64-detector row CT scanner (Aquilion 64; Toshiba Medical Systems) before the surgery. After obtaining unenhanced CT images, a total of 80–150 mL of the nonionic iodinated contrast material iopamidol (Bayer Healthcare, Osaka, Japan) was injected intravenously at a rate of 2.7–3.5 mL/s. Scanning was started 80 s after the injection. The imaging parameters were as follows: rotation time, 0.5 s; section thickness and intervals, 1 mm; beam collimation, 1 mm; pitch, 53; 120 kVp; 200 mAs; field of view, 35 cm2; matrix, 512 × 512; and voxel size, 0.68 × 0.68 × 1 mm3. Using these raw datasets, we obtained axial images with a slice thickness of 1 mm and an interval of 1 mm.
Preoperative N staging
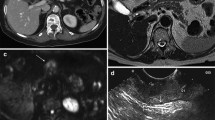
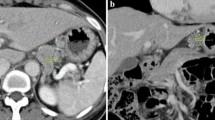
A specialized radiologist (H.O.) and a gastric surgeon with sufficient experience in diagnostic imaging (M.O.) who was blinded to the endoscopic findings performed the preoperative N staging using only the axial MDCT images. When there was a difference between the initial diagnoses of the two investigators, it was fixed by discussion. Previously validated criteria for diagnosing lymph node metastasis from gastric cancer [21–24] were referred. The regional lymph nodes were considered to be metastatic if they (1) had a short-axis diameter >8 mm (Fig. 1a); (2) were round and exhibited a central low-attenuation area, suggesting necrosis (Fig. 1a, b); and/or (3) exhibited clustering (three nodes or more) (Fig. 1a, c). Clustered nodes were staged as cN2 or cN3 according to the number of nodes estimated on the images.
a Image obtained in a 63-year-old man. Clustered (more than seven) lymph nodes were observed along the lesser curvature of the stomach (arrows). Some of them had a short-axis diameter >8 mm and round shape with a central low-attenuation area. The preoperative and postoperative N staging were cN3 and pN3 (35/55), respectively. The primary tumor was located in the upper third of the stomach, cT3 (pT3), type 2, 6.2 cm in size, and differentiated-type carcinoma. b Image obtained in a 58-year-old woman. One round-shaped lymph node with a central low-attenuation area was visualized along the left gastric artery (arrow). The preoperative and postoperative N staging were cN1 and pN1 (2/26), respectively. The primary tumor was located in the middle third of the stomach, cT2 (pT1b), type 0, 0.8 cm in size, and undifferentiated-type carcinoma. c Image obtained in a 37-year-old woman. The regional lymph nodes were all small (<8 mm) but were clustered (more than seven) along the lesser curvature of the stomach (arrows). The preoperative and postoperative N staging were cN3 and pN3 (25/42), respectively. The primary tumor was spreading circumferentially from the upper to lower third of the stomach, cT4a (pT4a), type 4, 16 cm in size, and undifferentiated-type carcinoma
Evaluation of diagnostic accuracy
The results of the preoperative N staging were compared with those for postoperative pathologic N staging. The overall diagnostic accuracy of the N staging, the accuracy for diagnosing lymph node metastasis (≥pN1), and the accuracy for diagnosing multiple lymph node metastases (≥pN2) were calculated.
Statistics
IBM Statistical Package for Social Science (SPSS) 20.0 (IBM, Corp., Armonk, NY, USA) was used for the statistical analyses. The Chi-square tests were applied to evaluate the associations of various tumor characteristics with preoperative over-/under-N staging. Multivariate logistic regression analyses were performed to identify the tumor characteristics independently associated with preoperative over-/under-N staging using variables with a P value of <0.20 in the Chi-square tests. P values of <0.05 were considered to indicate statistical significance.
Results
Clinicopathologic features
The clinicopathologic features of the 218 patients are shown in Table 1. The male-to-female ratio was 2.3 (151/67), and the median age was 64 years (range, 33–83 years). The middle third and lesser curvature of the stomach were the sites most commonly involved by the tumors. One hundred and eighty-two (83.5 %) patients had a macroscopic tumor type of 0, 2, or 3. One hundred and twenty-six (57.8 %) patients had undifferentiated-type carcinoma. Regarding the pT stage, pT2-4 accounted for 86.2 % (n = 188), while pT1 accounted for 13.8 % (n = 30). No severe adverse events related to the CT examinations occurred in the present series. The mean time interval between the CT examination and surgery was 22.5 days (standard deviation, 9.9 days).
Surgical procedures
The surgical procedures that were used for the 218 patients in this series are shown in Table 2. All the procedures were performed via laparotomy. A distal gastrectomy was performed in 143 patients. A total gastrectomy was performed in 75 patients, of whom 63 underwent a combined resection of the spleen. The median number of retrieved regional lymph nodes was 42 (range, 13–99). An R0 resection was achieved in 206 (94.5 %) patients. The factors precluding R0 resection were positive lavage cytology (n = 9), positive resection margin (n = 2), and intraoperatively found liver metastasis (n = 1).
Accuracy of preoperative N staging
The accuracy of preoperative N staging in the 218 patients is shown in Table 3. The distribution of the pN stage was as follows: pN3, n = 52; pN2, n = 39; pN1, n = 43; and pN0, n = 84. The overall accuracy of N staging was 46.3 % (101/218, 95 % confidence interval [CI]: 39.7–53.0), with the proportion of over- and under-N staging being 26.6 % (58/218, 95 % CI: 20.7–32.5) and 27.1 % (59/218, 95 % CI: 21.1–33.0), respectively. The accuracy according to the pN stage was as follows: pN3, 46.2 % (24/52, 95 % CI: 32.1–60.2); pN2, 53.8 % (21/39, 95 % CI: 37.5–70.2); pN1, 32.6 % (14/43, 95 % CI: 18.0–47.1); and pN0, 50.0 % (42/84, 95 % CI: 39.1–60.9).
The overall incidence of lymph node metastasis (≥pN1) was 61.5 % (134/218). The preoperative diagnostic sensitivity, specificity, and accuracy for ≥pN1 were 79.1 % (106/134, 95 % CI: 72.1–86.1), 50.0 % (42/84, 95 % CI: 39.1–60.9), and 67.9 % (148/218, 95 % CI: 61.6–74.1), respectively.
The overall incidence of multiple lymph node metastases (≥pN2) was 41.7 % (91/218). The preoperative diagnostic sensitivity, specificity, and accuracy for ≥pN2 were 80.2 % (73/91, 95 % CI: 71.9–88.6), 68.5 % (87/127, 95 % CI: 60.3–76.7), and 73.4 % (160/218, 95 % CI: 67.5–79.3), respectively.
The initial diagnoses of the two investigators were different in 43 (19.7 %) of the 218 patients. Of the 43 patients, 30 were finally diagnosed as having cN2 or cN3 disease.
Tumor characteristics associated with the preoperative N staging accuracy
The Chi-square tests showed that the macroscopic type and size of the primary tumor were significantly associated with preoperative over-N staging (P = 0.049 and 0.033, respectively). The macroscopic type was also significantly associated with under-N staging (P = 0.048) (Table 4). Preoperative over-N staging was most common among type 2 tumors and ≥6 cm-sized tumors, while under-N staging was most common among type 1/3 tumors. The incidence of lymph node metastasis (≥pN1) according to macroscopic tumor type was as follows: type 0, 40.0 % (24/60); type 1, 86.7 % (13/15); type 2, 65.3 % (47/72); type 3, 76.0 % (38/50); type 4, 61.5 % (8/13); and type 5, 50.0 % (4/8).
The multivariate analyses showed that macroscopic type 2 tumors and ≥6 cm-sized tumors were significantly associated with preoperative over-N staging (P = 0.025 and 0.035, respectively), while macroscopic type 1/3 tumors were significantly associated with under-N staging (P = 0.005) (Tables 5 and 6).
Discussion
In this study, we evaluated the use of 64-channel CE-MDCT for preoperative N staging according to the 7th (UICC) TNM Staging System in patients with resectable cT2-4 gastric carcinoma. The surgical procedures were performed uniformly via laparotomy based on the Japanese standards, with all patients undergoing a D2 lymphadenectomy. Although preoperative N staging with pinpoint accuracy was difficult, ≥pN2 disease was diagnosed with a reasonably high sensitivity and specificity. Thus, CE-MDCT appears to be useful for selecting patients who may benefit from neoadjuvant therapies.
Previously reported criteria for diagnosing lymph node metastasis from gastric cancer using MDCT have included (1) a short axis >8 mm [21, 22]; (2) a short axis (perigastric) >6 mm and a short axis (extraperigastric) >8 mm [23]; (3) a long axis ≥8 mm with marked enhancement [25]; and (4) a short axis (perigastric) >6 mm, a short axis (extraperigastric) >8 mm, and central necrosis or clustering (three nodes or more) regardless of size [24], the last of which is similar to ours. Because considerable differences exist in imaging techniques, image analyses, and baseline tumor stages among these studies, a direct comparative evaluation of the utility of each diagnostic criterion may be difficult. In the present study, we performed preoperative N staging using only axial CT images with a slice thickness of 1 mm exclusively in cT2-4 gastric carcinomas. In this context, our diagnostic criterion showed a similar bias toward over- and under-N staging, and thus was considered to be feasible.
Using axial CT images alone, Hasegawa et al. [22] reported a higher specificity (96.8 %) but a lower sensitivity (46.2 %) for lymph node metastasis, compared with our findings. This difference may be partly attributed to their use of a size criterion alone (short axis >8 mm) and of a slice thickness of 7 mm. In addition, more than half of their cohort had pT1 tumors with a low risk of lymph node metastasis, also presumably contributing to the difference in their results from ours. Using the 7th (UICC) TNM Staging System, Kim et al. [15] reported a higher overall N staging accuracy (63.2 %) than ours. Again, more than half of their cohort had pT1 tumors, which probably explains their superior result. In fact, a more advanced pathologic stage was shown to be associated with a probability of preoperative under-N staging [15]. Comparing the value of various diagnostic criteria in the same patient population using the same imaging techniques and image analyses may be a future task. It may help select the preferred criterion in that particular clinical situation.
One of the biggest problems is the difficulty in differentiating pN0 and pN1 disease. A future challenge will likely be the more precise detection of small-sized and small numbers of metastatic lymph nodes preoperatively using not only morphologic imaging, but also a combination of functional and morphologic imaging modalities, including positron emission tomography and/or magnetic resonance imaging [13, 26–31]. Sentinel node biopsy is another attractive diagnostic modality, but it is an invasive procedure and its potential efficacy and feasibility have only been shown for small cT1-2 tumors [32].
The macroscopic type and size of the primary tumor appears to affect the accuracy of preoperative N staging using CE-MDCT. In the present study, type 2 tumors and ≥6 cm-sized tumors were most susceptible to preoperative over-N staging presumably because of benign lymphadenopathy [33], which is often caused by coexisting ulcers. In contrast, type 3 tumors were susceptible to under-N staging, although they also accompany ulcers. The infiltrative nature of type 3 tumors may induce multiple metastases more frequently than the coexisting ulcers cause benign lymphadenopathy. We obtained another interesting result in that type 1 tumors were found to be susceptible to under-N staging. This result may be partly attributed to the low incidence of ulcer-related lymphadenopathy in this tumor type. In our clinical practice, each attending gastric surgeon, who is open to the findings of all preoperative examinations, determines the final preoperative N stage after referring the radiologist’s diagnosis. Modifications of the diagnostic criteria in consideration of the macroscopic type and size of the primary tumor may provide better N staging accuracy in cT2-4 gastric carcinomas.
One of the limitations of our study is that the preoperative N staging was performed by only two investigators. Despite the use of pre-specified criteria, a limited number of image reviewers can result in a bias. In particular, the diagnosis of clustered nodes might vary according to the reviewer. Another inevitable limitation is that the clinical and pathological findings were not matched at the level of single node or node stations. In addition, the substantial and dispersion of the time interval between CT examination and surgery are a potential source of bias. In consideration of these limitations, we should interpret the study results cautiously.
References
Parkin DM (2004) International variation. Oncogene 23:6329–6340
Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T et al (2000) Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 3:219–225
Hirasawa T, Gotoda T, Miyata S, Kato Y, Shimoda T, Taniguchi H et al (2009) Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer 12:148–152
Katai H, Morita S, Saka M, Taniguchi H, Fukagawa T (2010) Long-term outcome after proximal gastrectomy with jejunal interposition for suspected early cancer in the upper third of the stomach. Br J Surg 97:558–562
Morita S, Katai H, Saka M, Fukagawa T, Sano T, Sasako M (2008) Outcome of pylorus-preserving gastrectomy for early gastric cancer. Br J Surg 95:1131–1135
Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, Japan Clinical Oncology Group et al (2008) D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med 359:453–462
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, MAGIC Trial Participants et al (2006) Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355:11–20
D’Ugo D, Rausei S, Biondi A, Persiani R (2009) Preoperative treatment and surgery in gastric cancer: friends or foes? Lancet Oncol 10:191–195
Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G et al (2011) Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 29:1715–1721
Siewert JR, Böttcher K, Stein HJ, Roder JD (1998) Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric cancer Study. Ann Surg 228:449–461
Park SR, Kim MJ, Ryu KW, Lee JH, Lee JS, Nam BH et al (2010) Prognostic value of preoperative clinical staging assessed by computed tomography in resectable gastric cancer patients: a viewpoint in the era of preoperative treatment. Ann Surg 251:428–435
Kwee RM, Kwee TC (2007) Imaging in local staging of gastric cancer: a systematic review. J Clin Oncol 25:2107–2116
Kwee RM, Kwee TC (2009) Imaging in assessing lymph node status in gastric cancer. Gastric Cancer 12:6–22
Sobin LH, Gospodarowicz MK, Wittekind CH (2009) TNM classification of malignant tumours, 7th edn. Wiley-Blackwell, New York
Kim SH, Kim JJ, Lee JS, Kim SH, Kim BS, Maeng YH et al (2013) Preoperative N staging of gastric cancer by stomach protocol computed tomography. J Gastric Cancer 13:149–156
Matsuki M, Tanikake M, Kani H, Tatsugami F, Kanazawa S, Kanamoto T et al (2006) Dual-phase 3D CT angiography during a single breath-hold using 16-MDCT: assessment of vascular anatomy before laparoscopic gastrectomy. AJR Am J Roentgenol 186:1079–1085
Takiguchi S, Sekimoto M, Fujiwara Y, Yasuda T, Yano M, Hori M et al (2004) Laparoscopic lymph node dissection for gastric cancer with intraoperative navigation using three-dimensional angio computed tomography images reconstructed as laparoscopic view. Surg Endosc 18:106–110
Horton KM, Fishman EK (2003) Current role of CT in imaging of the stomach. Radiographics 23:75–87
Japanese Gastric Cancer Association (2011) Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14:101–112
Association Japanese Gastric Cancer (2011) Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 14:113–123
Kim HJ, Kim AY, Oh ST, Kim JS, Kim KW, Kim PN et al (2005) Gastric cancer staging at multi-detector row CT gastrography: comparison of transverse and volumetric CT scanning. Radiology 236:879–885
Hasegawa S, Yoshikawa T, Shirai J, Fujikawa H, Cho H, Doiuchi T et al (2013) A prospective validation study to diagnose serosal invasion and nodal metastases of gastric cancer by multidetector-row CT. Ann Surg Oncol 20:2016–2022
Hur J, Park MS, Lee JH, Lim JS, Yu JS, Hong YJ et al (2006) Diagnostic accuracy of multidetector row computed tomography in T and N staging of gastric cancer with histopathologic correlation. J Comput Assist Tomogr 30:372–377
Yan C, Zhu ZG, Yan M, Zhang H, Pan ZL, Chen J et al (2009) Value of multidetector-row computed tomography in the preoperative T and N staging of gastric carcinoma: a large-scale Chinese study. J Surg Oncol 100:205–214
Chen CY, Hsu JS, Wu DC, Kang WY, Hsieh JS, Jaw TS et al (2007) Gastric cancer: preoperative local staging with 3D multi-detector row CT—correlation with surgical and histopathologic results. Radiology 242:472–482
Staniuk T, Zegarski W, Małkowski B, Jankowski M, Klag M, Pietrzak T (2013) Evaluation of FLT-PET/CT usefulness in diagnosis and qualification for surgical treatment of gastric cancer. Contemp Oncol (Pozn) 17:165–170
Jeong J, Cho I, Kong E, Chun K, Jang B, Kim T et al (2013) Evaluation of hybrid PET/CT gastrography in gastric cancer. Nuklearmedizin 52:107–112
Smyth E, Schöder H, Strong VE, Capanu M, Kelsen DP, Coit DG et al (2012) A prospective evaluation of the utility of 2-deoxy-2-[(18) F]fluoro-d-glucose positron emission tomography and computed tomography in staging locally advanced gastric cancer. Cancer 118:5481–5488
Zieker D, Königsrainer I, Weinreich J, Beckert S, Glatzle J, Nieselt K et al (2010) Phosphoglycerate kinase 1 promoting tumor progression and metastasis in gastric cancer—detected in a tumor mouse model using positron emission tomography/magnetic resonance imaging. Cell Physiol Biochem 26:147–154
Will O, Purkayastha S, Chan C, Athanasiou T, Darzi AW, Gedroyc W et al (2006) Diagnostic precision of nanoparticle-enhanced MRI for lymph-node metastases: a meta-analysis. Lancet Oncol 7:52–60
Herborn CU, Lauenstein TC, Vogt FM, Lauffer RB, Debatin JF, Ruehm SG (2002) Interstitial MR lymphography with MS-325: characterization of normal and tumor-invaded lymph nodes in a rabbit model. AJR Am J Roentgenol 179:1567–1572
Kitagawa Y, Takeuchi H, Takagi Y, Natsugoe S, Terashima M, Murakami N et al (2013) Sentinel node mapping for gastric cancer: a prospective multicenter trial in Japan. J Clin Oncol 31:3704–3710
Park HS, Kim YJ, Ko SY, Yoo MW, Lee KY, Jung SI et al (2012) Benign regional lymph nodes in gastric cancer on multidetector row CT. Acta Radiol 53:501–507
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All the authors report no conflicts of interest relevant to this article.
Rights and permissions
About this article
Cite this article
Ohashi, M., Morita, S., Fukagawa, T. et al. Evaluation of 64-Channel Contrast-Enhanced Multi-detector Row Computed Tomography for Preoperative N Staging in cT2-4 Gastric Carcinoma. World J Surg 40, 165–171 (2016). https://doi.org/10.1007/s00268-015-3318-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-015-3318-8