Abstract
Background
Totally implantable venous access ports (TIVAP) are eventually explanted for various reasons, related or unrelated to the implantation technique used. Having more information on long-term explantation would help improve placement techniques.
Methods
From a series of 1572 cancer patients who had TIVAPs implanted in our center with the cutdown technique or Seldinger technique, we studied the 542 patients who returned to us to have their TIVAP explanted after 70 days or more. As outcome measures we distinguished between TIVAPs explanted for long-term complications (infection, catheter-, reservoir-, and patient-related complications) and TIVAPs no longer needed. Univariate and multivariate analyses were run to investigate the reasons for explantation and their possible correlation with implantation techniques.
Results
The most common reason for explantation was infection (47.6 %), followed by catheter-related (20.8 %), patient-related (14.7 %), and reservoir-related complications (4.7 %). In the remaining 12.2 % of cases, the TIVAP was explanted complication free after the planned treatments ended. Infection correlated closely with longer TIVAP use. Univariate and multivariate analyses identified the Seldinger technique as a major risk factor for venous thrombosis and catheter dislocation.
Conclusions
The need for long-term TIVAP explantation in about one-third of cancer patients is related to the implantation techniques used.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As devices for delivering long-term chemotherapy in patients with cancer, totally implantable venous access ports (TIVAPs) have now gained standard acceptance. Compared with external central venous catheters, TIVAPs provide simple and more effective management, offer patients a better quality of life and reduce infective and thrombotic catheter-related complications [1, 2]. The two main methods used for TIVAP implantation are the cutdown technique (CDT) and the Seldinger technique (ST). The first involves surgically preparing the cephalic vein at the deltopectoral groove whereas the second requires subclavian vein or internal jugular vein puncture, with or without ultrasound guidance [3–6].
Because no definitive conclusions have yet shown that one insertion technique has advantages over the other in reducing early TIVAP-related complications, surgeons decide which technique to use according to their personal preferences [7, 8]. Whichever placement technique is used TIVAPs eventually have to be explanted for various reasons [9, 10]. Possible help in differentiating how the two implantation techniques influence long-term outcome comes from analyzing the reasons for explantation. TIVAPs are often explanted late in their lifetime (years after being implanted) because complications including infection or vein thrombosis make them no longer usable [11], patients choose to have them removed or they are no longer needed. Knowing whether these late adverse events depend on the implantation technique would help in choosing the most appropriate technique.
Our aim in this retrospective study was to compare the causes leading to long-term explantation (more than 3 months after implantation) in cancer patients whose TIVAPs were also placed in our department with the CDT or ST. We sought to identify possible correlations between the implantation techniques used and the reasons necessitating long-term removal.
Methods
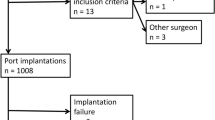
From a series of 1572 consecutive cancer patients who had their TIVAP implanted with the CDT or ST from February 2000 to December 2010 in our center, we studied the 542 (34.5 %) patients who returned for long-term explantation up to 31 December 2013. To exclude early and late complications, we included in the study only patients whose TIVAPs were explanted more than 70 days after implantation. We also excluded deceased patients, patients who had their TIVAPs explanted in other centers, and those who had undergone a previous implantation (Fig. 1).
All TIVAPs were implanted in day surgery in the same operating room under local anesthesia (2 % carbocaine, maximum dose 10 ml) and intravenous antibiotic prophylaxis by two experienced surgical teams, each comprising two expert surgeons, all of whom had already done more than 200 implants, one team specialized in the CDT and the other in the ST. The two surgical teams implanted TIVAPs in all the cancer patients referred for implantation without selection criteria. All patients were implanted with the same type of TIVAP device “Celsite” by B/Braun with a silicone catheter. For CDT, the central venous catheter (CVC) caliber was chosen according to the cephalic vein diameter. ST catheters were mainly 6.5 French. For the CDT, the left cephalic vein was isolated at the ipsilateral deltopectoral groove. The right side was chosen for left-handed patients and for those who had undergone aggressive surgery in areas adjacent to the left deltopectoral area. If the cephalic vein proved impossible to catheterize we proceeded to a Seldinger ipsilateral direct subclavian vein puncture. The preferred choice for the ST was generally a right-sided percutaneous subclavian vein puncture without ultrasound guidance. If this technique failed, the TIVAP was implanted through an internal jugular vein access under ultrasound guidance. During the procedure, a radiographic image was taken to check that the catheter tip was correctly placed in the superior vena cava.
All TIVAPS were explanted with patients under local anesthesia. When analyzing the reasons for explantation, as outcome measures in TIVAPs studied according to the placement technique used we distinguished between TIVAPs explanted owing to long-term complications (infection, catheter-, reservoir-, and patient-related complications) and TIVAPs no longer needed. In patients in whom TIVAPs were explanted for suspected infection, manifested during drug infusion with fever and chills, the bacterial population was verified by testing the explanted TIVAP microbiologically or by blood culture or both. TIVAP lifetimes were calculated in months elapsing between implantation and explantation.
Statistical analysis
Statistical differences between groups were calculated with the Chi square test (Χ 2 test). Multiple regression analyses were used to verify correlations between the two implantation techniques and long-term complications responsible for explantation. One-way analysis of variance (ANOVA) was run to compare TIVAP lifetime in the two groups. P values ≤ 0.05 were considered to indicate statistical significance. All data were analyzed with the NCSS statistical software package.
Results
Of the 542 patients who returned to our center to have their device explanted, 368 had TIVAP implanted with the CDT and 174 with the ST (Table 1). TIVAPs had a similar lifetime in the two groups (38.1 vs. 36.2 months). The most common reason for explantation was infection (47.6 %), followed by catheter-related (20.8 %), reservoir-related (4.7 %), and patient-related complications (14.7 %). In 12.2 % of the patients overall, TIVAPs were explanted because they were no longer needed (Table 2). Infectious complications led to port removal more often, though not significantly more often in TIVAPs implanted with the CDT than with the ST. In 69 of the 258 TIVAPs explanted for infection, microbiological testing identified bacterial (88.4 %) or fungal growth (11.6 %). The most common bacteria was Staphylococcus aureus and the most common fungus was Candida albicans.
Among catheter-related complications, a frequent reason for explantation was vein thrombosis. The incidence differed significantly between the two placement techniques and thrombosis developed more frequently in TIVAPs implanted by the ST than by the CDT (16.7 vs. 10.3 %; P = 0.03 by Χ 2 test). Multiple regression analysis confirmed that catheter-related vein thrombosis correlated significantly with the catheter ≥8.5 French in diameter and direct right subclavian vein puncture by the ST (Table 3).
Catheter-related complications also included dislocation, when the catheter tip migrated into the contralateral subclavian vein or into the internal jugular vein. This complication developed more frequently in patients whose TIVAPs were implanted with the ST than after CDT (4.6 vs. 0.3 %; P < 0.001 by Χ 2 test) and arose only after direct subclavian vein puncture and never after jugular vein access. Multiple regression analysis confirmed the difference specifying that catheter dislocation correlated significantly with the ST by subclavian vein access (Table 3). In 28 of the 542 patients (5.2 %), TIVAPs were removed because the catheter occluded; no significant difference was found in the incidence of this complication between the two implantation techniques. A severe catheter-related complication was the pinch-off syndrome [12]. This adverse event arose in 5.3 % of the patients (mean TIVAP lifetime 46.8 months) whose TIVAPs were placed by subclavian puncture access, the only placement technique at risk for this complication. In these patients, explantation entailed removing the migrated endovascular catheter segment with a radiologic endovascular technique.
Reservoir-related complications included port dislocation or dehiscence involving the skin overlying the reservoir and developed in 14 patients; no significant difference was found in the incidence of this complication between the two implantation techniques (Table 2).
Of the 542 patients whose TIVAPs were explanted, 80 chose to have them removed. No significant difference was found between removal rates in the CDT and ST groups. Patients’ decisions on removal reflected subjective disturbances (discomfort, pain, difficulty in moving the neck or shoulder, aesthetic problems). All the patients who wanted their TIVAPs removed for aesthetic reasons (visible subcutaneous catheter tunneling or reservoir pocket) originally had them implanted through direct internal jugular vein puncture. In 12.2 % of the patients overall, TIVAPs were explanted because they were no longer needed. Removal rates for this reason were not significantly higher in the CDT than in the ST placement group.
Discussion
A strong point in our study design is that by analyzing the causes leading to long-term TIVAP explantation in cancer patients we extend current knowledge showing that TIVAPs can have a long lifetime, even up to 12 years. Ideally TIVAPs for cancer patients should remain efficient and complication-free over time and should be removed when therapeutic programs end. In practice, only 12.2 % of the patients we studied had their TIVAPs removed because the therapeutic program ended. The remaining 87.8 % had them removed in the long term because various adverse events arose. In our series, the main reason for long-term explantation was infection (47.6 %) as others have reported [10, 11]. Infective complications were unrelated to the implant technique (50.3 vs. 42 %). TIVAP infections are multifactorial events related to the patient’s clinical condition (immunocompetence, performance status, blood count), the tumor type requiring therapy [11] and, most important, increased handling [13]. The high infection rate in our cancer patients depended on the long TIVAP survival (CDT 38.1 and ST 36.2 months) and presumably on increased risks related to lengthy management.
When we analyzed catheter-related TIVAP complications, we found that thrombosis developed less often in TIVAPs implanted by the CDT than after the ST (10.3 vs. 16.7 %, P = 0.03 by Χ 2 test). This difference could be related to minor venous trauma and less complex manoeuvers needed to insert the catheter in the cephalic vein with the CDT technique than with the ST in the subclavian or internal jugular vein, a procedure that needs at least 4 different passages through the vein (needle, guide-wire, dilator, and catheter). Even when done under ultrasound guidance, repeated maneuvers can damage the vein wall and increase the thrombotic risk [5]. Neither repeated maneuvers nor the implantation technique seem likely to be responsible for thrombosis arising in the long term (even years after the implant). Equally important, our multivariate analysis shows right subclavian vein access as a further significant risk factor for thrombosis (P = 0.002) (Table 3). We, therefore, attribute this risk to the observation that most patients are right-handed and hence the predominant motor activity in the right arm can lead to repeated trauma at the point where the catheter enters the subclavian vein.
Another catheter-related complication arising in the long-term was the pinch-off syndrome. This adverse event developed in more than 5 % of the patients and arose within years after implantation. As expected, all these patients’ TIVAPs were implanted by the ST with subclavian vein access. These findings are hard to compare with the literature insofar as published case series analyze complications (often misleadingly identified as late) arising up to a maximum of 60–70 days after implantation whereas the pinch-off syndrome arises months or years after implantation [14]. Another problem that makes findings for this complication hard to interpret is that the pinch-off syndrome is described exclusively in case reports unrelated to the case series from which they originated [15]. Removing the catheter segment that has migrated into the vascular system requires interventional radiology and hospital admission, events that worsen risks for the patient and increase hospital costs [16].
TIVAPs may also have to be removed for other catheter-related problems. In our series, in nine patients (1.7 %) TIVAPs had to be removed because the catheter dislocated. Of these nine patients, eight had TIVAPs implanted in subclavian vein (ST) and only one had TIVAPs implanted with CDT (P < 0.001 by Χ 2 test). In four of these eight cases, the catheter was partially expelled from the vein and rolled up around the reservoir in the subcutaneous pocket. Our experience suggests that these adverse events reflect two technical shortcomings. First, direct puncture placement foresees no system for anchoring the catheter to the vein. Hence, after a subclavian vein ST implant any event that temporarily increases superior caval vein pressure (including repeated coughing, straining, and vomiting) can progressively expel the catheter and dislocate it from its normal position in the reservoir pocket. In the other four patients, the catheter tip dislocated into the contralateral subclavian or jugular vein, mainly owing to increased venous pressure or catheter shortness. This complication never arose in patients with TIVAPs implanted in the jugular vein, presumably because jugular vein catheters are unlikely to dislocate given that the subcutaneous tunnel is invariably longer than the subclavian tract and the entire endovascular tract is linear.
When we investigated reservoir-related complications, we found that in 8 patients who underwent both ST and CDT, TIVAPs had to be explanted because skin dehiscence and local sepsis developed over the reservoir. The weight loss typical in patients with cancer presumably leads to subcutaneous fat thinning causing the reservoir to adhere to the skin. Repeated membrane puncture or decubitus from prolonged use of the gripper needles create skin trauma with progressive tissue loss, bacterial contamination causing pocket infection, and reservoir expulsion [11, 17]. Other reservoir-related complications independent from the implantation technique included 6 patients who had their TIVAPs explanted because the reservoir turned over and dislocated thus making the system unusable. The last reservoir-related complication was drug extravasation (2.2 %), exclusively related to poor TIVAP management.
A major, unexpected finding was that even though their TIVAPs were functioning properly, 80 patients (15.5 % in the CDT vs. 13.2 % in the ST group) asked to have them removed because of discomfort (local pain, feeling of traction, irritation when moving the arm, shoulder or neck, or aesthetic reasons). Of the 23 patients in the ST group who had jugular vein TIVAPs (Table 2), 18 asked to have them removed chiefly for aesthetic problems. The main reasons why women found the catheter intolerable were its lengthy subcutaneous tunneling in the neck, and passage over the clavicle. For two reasons, this technique should be reserved to patients with a rich subcutaneous fat layer: first, because the subcutaneous fat covers the catheter track; second, inserting the subcutaneous pocket in the anterior median pectoralis region rather than the deltopectoral groove makes it easier to find the reservoir.
Conclusion
In our study, the major long-term TIVAP complication was infection probably related to lengthy management and linked to long TIVAP lifetimes, regardless of the implant technique used. The other reasons necessitating long-term TIVAP explantation correlated with the implantation technique used. The rates for catheter-related complications (venous thrombosis, dislocation, pinch-off syndrome) correlated significantly with ST placement. Many women with TIVAPs implanted by the ST in the jugular vein wanted their devices removed for aesthetic reasons. These findings merit confirmation in a prospective multicenter study designed to obtain information on long-term TIVAP outcomes, though these studies take several years to complete.
TIVAP management must be entrusted to experienced staff, and to reduce the high risk of complications resulting from poor management TIVAP procedures must follow specific protocols. The choice of site and implantation technique should take into account the individual patient’s anatomic and aesthetic characteristics. If TIVAPs are correctly placed and systems properly managed, patients can use them for a decade or more.
References
Bow EJ, Kilpatrick MG, Clinch JJ et al (1999) Totally implantable venous access ports systems for patients receiving chemotherapy for solid tissue malignancies: a randomized controlled clinical trial examining the safety, efficacy, costs, and impact on quality of life. J Clin Oncol 17(4):1267
Silver DF, Hempling RE, Recio FO et al (1998) Complications related to indwelling caval catheters on a gynecologic oncology service. Gynecol Oncol 70(3):329–333
Povoski SP (2000) A prospective analysis of the cephalic vein cutdown approach for chronic indwelling central venous access in 100 consecutive cancer patients. Ann Surg Oncol 7(7):496–502
Seldinger SI (1953) Catheter replacement of the needle in percutaneous arteriography; a new technique. Acta Radiol 39:368–376
Teichgräber UK, Kausche S, Nagel SN et al (2011) Outcome analysis in 3,160 implantations of radiologically guided placements of totally implantable central venous port systems. Eur Radiol 21(6):1224–1232
Cavanna L, Civardi G, Vallisa D et al (2010) Ultrasound-guided central venous catheterization in cancer patients improves the success rate of cannulation and reduces mechanical complications: a prospective observational study of 1,978 consecutive catheterizations. World J Surg Oncol 19(8):91
Knebel P, Lopez-Benitez R, Fischer L et al (2011) Insertion of totally implantable venous access devices: an expertise-based, randomized, controlled trial (NCT00600444). Ann Surg 253(6):1111–1117
Nocito A, Wildi S, Rufibach K et al (2009) Randomized clinical trial comparing venous cutdown with the Seldinger technique for placement of implantable venous access ports. Br J Surg 96(10):1129–1134
Di Carlo I, Cordio S, La Greca G et al (2001) Totally implantable venous access devices implanted surgically: a retrospective study on early and late complications. Arch Surg 136(9):1050–1053
Fischer L, Knebel P, Schröder S et al (2008) Reasons for explantation of totally implantable access ports: a multivariate analysis of 385 consecutive patients. Ann Surg Oncol 15(4):1124–1129
Kurul S, Saip P, Aydin T (2002) Totally implantable venous-access ports: local problems and extravasation injury. Lancet Oncol 3(11):684–692
Hinke DH, Zandt-Stastny DA, Goodman LR et al (1990) Pinch-off syndrome: a complication of implantable subclavian venous access devices. Radiology 177(2):353–356
Lebeaux D, Fernández-Hidalgo N, Chauhan A et al (2014) Management of infections related to totally implantable venous-access ports: challenges and perspectives. Lancet Infect Dis 14(2):146–159
Mirza B, Vanek VW, Kupensky DT (2004) Pinch-off syndrome: case report and collective review of the literature. Am Surg 70(7):635–644
Di Carlo I, Fisichella P, Russello D et al (2000) Catheter fracture and cardiac migration: a rare complication of totally implantable venous devices. J Surg Oncol 73(3):172–173
Biffi R, de Braud F, Orsi F et al (1998) Totally implantable central venous access ports for long-term chemotherapy. A prospective study analyzing complications and costs of 333 devices with a minimum follow-up of 180 days. Ann Oncol 9(7):767–773
Bassi KK, Giri AK, Pattanayak M, Abraham SW et al (2012) Totally implantable venous access ports: retrospective review of long-term complications in 81 patients. Indian J Cancer 49(1):114–118
Author contributions
Study conception and design: Biacchi, Di Giorgio. Acquisition of data: Accarpio, Cardi, Impagnatiello, Fouad Atta. Analysis and interpretation of data: Sammartino, Sapienza, De Cesare. Drafting of manuscript: Biacchi, Sibio. Critical revision and final approval: Di Giorgio, Biacchi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest and received no financial support.
Rights and permissions
About this article
Cite this article
Biacchi, D., Sammartino, P., Sibio, S. et al. Does the Implantation Technique for Totally Implantable Venous Access Ports (TIVAPs) Influence Long-Term Outcome?. World J Surg 40, 284–290 (2016). https://doi.org/10.1007/s00268-015-3233-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-015-3233-z