Abstract
Purpose
Totally implantable venous access ports (TIVAP) have been widely used for many years in the management of patients suffering from cancer. The implantation and long-term use of TIVAPs are associated with mechanical, thrombotic, and infectious complications. This is the first exhaustive prospective study of all complications occurring in a whole population on long-term follow-up and therefore allows an objective assessment to be made of the safety of TIVAPs.
Methods
We carried out a prospective single-center observational study. All adult patients with cancer who had a TIVAP implanted between January 1 and December 31, 2006 were registered. Early and late complications were recorded until the removal of the device, the patient’s death, or until December 31, 2013. Exhaustive data concerning patients and TIVAP was recorded at time of implantation.
Results
Four hundred and ninety-three TIVAPs were implanted in 483 adult cancer patients and were followed during a period from 1 to 94 months (median = 18 months) representing a global quantity of 367,359 catheter-days. Eighty-seven complications were recorded (0.237/1000 catheter-days), including 37 infections (0.101/1000 catheter-days), 17 thrombotic events (0.046/1000 catheter-days), and 9 extravasations. Out of the 87 events, 62 (71.3%) occurred during the first year after implantation. Events were therefore extremely rare after 1 year. Thromboembolic and infectious complications were rare and no risk factors for these were found.
Conclusions
This study demonstrates excellent tolerability, with only occasional complications. Most of these occurred during the year following implantation. A TIVAP may also be left in place for an extremely long time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of totally implantable venous access ports (TIVAPs) has become routine in the management of patients suffering from cancer as they facilitate administration of anti-cancer agents, blood products, antibiotics, and parenteral nutrition. Review of the literature shows that their implantation and use are associated with complications such as pneumothorax, catheter rupture, and the port casing turning around. The implantation of a TIVAP into a subclavian vein may result in a perioperative complication rate of up to 12% [1, 2]. The most common complications are thrombotic and infectious, with reported rates respectively ranging from 0.3 to 28% [3], and from 5.6 to 8% [4]. These complications are life threatening to the patient, cause distress, and result in delays in continuing chemotherapy.
Few prospective studies have examined all of the thrombotic, infectious, and other complications (pneumothorax, catheter rupture, port casing turning around, etc.) of TIVAPs [5, 6]. These studies have mainly focused on infectious or thromboembolic complications, and many have been retrospective with small patient numbers and generally short follow-up periods. It is therefore difficult to obtain an estimate of the overall complications associated with implantation and long-term use of TIVAPs.
We conducted a prospective study of all totally implantable venous access ports implanted during a 1-year period in our cancer center with the aim of describing the acute and late complications and identifying the associated risk factors.
Patients and methods
We conducted a prospective observational study in our cancer center (Clinique Victor Hugo, Institut Inter-régional de Cancérologie, Le Mans, France). The Clinique Victor Hugo is a comprehensive cancer center working in a network with the community hospitals. Staffs in these community hospitals have been trained by Clinique Victor Hugo staff in the management of TIVAPs for the administration of chemotherapy and supportive care. In 2006, a total of 2343 new patients were treated at the Clinique Victor Hugo in 2006 from a recruitment catchment area of 1 million people. All totally implantable venous access ports implanted in the chest or upper limbs in our patients between January 1 and December 31, 2006 were included. We consider that there are no contraindications to TIVAP implantation. The very occasional cases of femoral TIVAP were not included in our study as these raise specific problems.
Implantation and use of the TIVAPs
The decision to implant a TIVAP was made by the medical oncologist and/or supportive care specialist and a written request was sent to the surgeon. All of the TIVAPs were implanted by one of the 12 experienced general or vascular surgeons in six public or private hospitals belonging to our comprehensive cancer center network. They were implanted in an operating room under local or general anesthesia using standard technique. In some cases, the implantation of a TIVAP was combined with another surgical procedure (tumor biopsy or excision). The choice of TIVAP, implantation technique, site (subclavian, internal jugular, or basilic vein), and side were decided by the surgeon in agreement with patient’s choice. Only polyurethane catheters were implanted. Correct implantation was confirmed by perioperative radioscopy, post-operative pulmonary radiography, or both of these techniques. Heparin was not used for the prevention of intraluminal thrombosis during and after operation period. No prophylactic anticoagulant or antibiotic therapy was given.
All of the patients received their chemotherapy in our center. The standard protocol whenever a TIVAP was used was to confirm patency of the device by injecting 10 ml of physiological saline into the TIVAP, to proceed to planned injection then to rinse the catheter with 10 ml of physiological saline. When the TIVAP was used several days in succession, the needle and infusion tubing were left in place overnight and changed every 5 days. The TIVAPs could be used at home for bisphosphonate infusions or parenteral nutrition. In this situation, the nurses followed the same procedures used in our center. The TIVAPs were not intended for routine blood sampling although blood cultures to document a TIVAP infection were permitted. The TIVAPs were not routinely rinsed or checked for patency when they were not in use.
Data collection
The data recorded at inclusion were patient age, gender, past thromboembolic history, use of platelet antiaggregant or anticoagulant therapy, tumor site and staging of the cancer, type of use of the TIVAP (adjuvant chemotherapy, curative, or metastatic chemotherapy) order number of the TIVAP (1st TIVAP, 2nd TIVAP, or >2nd TIVAP), type of TIVAP, implantation technique, number of attempts (1; >1), and methods used to check correct positioning.
We drew up a specific declaration form for external or internal professionals to notify adverse events considered to be certainly, probably, or possibly related to the TIVAP to be used for reporting purposes and for the analysis. All TIVAPs were followed up from the implantation date until removal, death of the patient, or until December 31, 2013.
At the date of the design of the study, institutional review board was not mandatory because it was an observational non-interventional study.
Study outcomes
Catheter lifespans started from the implantation of the catheter until its removal or death of the patient, last follow-up, or December 31, 2013, whichever came first. These were measured in catheter-days.
Thromboembolism
Catheter-related thrombosis, deep vein thrombosis, and pulmonary embolism were diagnosed according to usual procedures.
Catheter occlusion was defined as the inability to flush a catheter. Causes were either an adherent fibrin and collagen coating covering the catheter tip or thrombosis of the catheterized vein (catheter-related thrombosis).
Catheter-related thrombosis was confirmed by ultrasonography and/or CT angiography. Previously published criteria were used: noncompressibility of a venous segment of the upper arm or the internal jugular vein on venous ultrasonography, absent or reduced flow on Doppler imaging with failure to augment on compression of the arm or lack of respiratory variation and the presence of echogenic material compatible with thrombus in the arm or central venous vasculature on real-time imaging, or the presence of intraluminal filling defect in two or more views seen in a venous segment of the arm or central venous vasculature on CT scan [7]. Systematic screening for catheter-related thrombosis in asymptomatic patients was not performed.
Symptomatic pulmonary embolism was diagnosed in patients with compatible symptoms if one or more of the following criteria were documented: intraluminal filling defect of a lobar artery or more proximal pulmonary arterial vasculature on spiral CT scan and/or a high-probability ventilation-perfusion lung scan [8]. Patients who developed a pulmonary embolism from lower limb venous thrombosis and those who developed pulmonary embolism without thrombosis in the vein in which the TIVAP had been implanted were not considered as events for this study.
Patients with catheter-related thrombosis were treated with anticoagulant therapy (mostly low molecular weight heparin) until complete regression of thrombosis on ultrasonography for at least 3 months. Catheter removal was encouraged as soon as possible. The removal of the catheter was discussed on an individual basis for palliative care patients.
Patients with pulmonary embolism were treated with anticoagulant therapy for at least 6 months. Low molecular weight heparins were preferred when patients were receiving chemotherapy (in view of the risk of drug interactions). A switch to a vitamin K antagonist was recommended once the chemotherapy had ended.
Adherent coating of fibrin and collagen was treated by flushing the TIVAP with urokinase (25,000 IU/ml).
Infection
The center’s policy if signs of infection were present (fever, chills, inflammation of the TIVAP cavity or scar, shock, etc.) was to take three blood cultures from the TIVAP and three blood cultures from a peripheral vein. The TIVAP was deemed to be infected if at least two of the blood cultures taken from the TIVAP returned the same microbiological documentation, regardless of the result of the blood cultures taken from a peripheral vein.
TIVAP infection could be confirmed by culturing the port if it was removed although the TIVAP culture procedures were not standardized: the catheter’s room was cultured in a culture medium, the catheter cultured in liquid medium or rolled onto a Petri dish.
Removal of the TIVAP
The removal of the TIVAP was proposed 12 months after the end of adjuvant chemotherapy or after the end of curative chemotherapy if no tumor recurrence had occurred.
The TIVAPs were routinely removed if they had become infected or if the patient developed a Pseudomonas, Staphylococcus aureus, or fungal septicemia and in cases of infectious endocarditis documented on echocardiography. If the infection involved another organism, an attempt was made to continue with the implanted TIVAP using dual antibiotic therapy tailored to the antibiotic sensitivity profile. The TIVAP was removed if blood cultures taken 48 h after starting antibiotic therapy were still positive. The decision to remove TIVAPs from patients on late stage palliative care was made on an individual case basis. If a TIVAP was still essential after one had been removed because of a complication, our recommendation was to implant another TIVAP on the contralateral side whenever possible.
Statistical analysis
This study was non-interventional and was conducted in accordance with current regulations. Consenting patients were enrolled at the time their TIVAP was implanted. Preselected baseline characteristics were assessed as potential risk factors.
Quantitative parameters were described as median (range—IQR) and qualitative ones as frequency of their respective modalities. Event-free survival was calculated by using Kaplan-Meier method. The primary end-point was the probability of thrombotic or infectious complication. Time to event was defined as the delay between catheter implantation and catheter ablation because of thrombotic or infectious complication (or last visit if no such event).
Univariate analysis of symptomatic complication by each potential risk factor was performed using logrank test (if qualitative) or univariate Cox (if quantitative). We then used a multivariate stepwise backward Cox regression model to simultaneously assess the relationship between baseline factors and the occurrence delay of developing a thrombotic or infectious complication.
All parameters with p < 0.15 at the univariate step were entered in the full model. Cox PH assumption will be verified (graphically and using Schoenfeld residuals) on each tested parameter before stepwise and on the final model after stepwise elimination.
All analyses will be two-sided, with p-significance at 5%, done using STATA 13.1 Special Edition (StataCorp, College Station, Texas, USA).
Results
Characteristics of the patients and TIVAPs (Tables 1 and 2)
Four hundred and ninety-three TIVAPs were implanted in 483 patients between January 1 and December 31, 2006. Ten patients received two TIVAPs in 2006 because of complications occurring during the year 2006, which required the removal of their TIVAP and implantation of a second device. Average patient age was 62.6 years old. Seventy-one patients (14.7%) had a past history of thromboembolic disease, and 11 (2.2%) had a past history of hemorrhage at the time of implantation. Thirty-five patients (7.2%) were receiving Vitamin K antagonists, 34 (7%) were receiving platelet antiaggregants, 9 (1.8%) were receiving preventative dose low molecular weight heparin (LMWH) (1.8%), and 1 (0.2%) was receiving curative dose LMWH. The patients were being treated for breast (25.9%), colorectal (16.6%), or lung cancer (8.28%). Patient characteristics, indications for chemotherapy, and types of TIVAPs are summarized in Tables 1, 2, and 3.
Follow-up and lifespan of the TIVAPs
Median patient follow-up was 30 months (range 1–94) and median follow-up of the TIVAPs was 18 months (range 1–94), i.e., a global follow-up of 367,359 catheter-days (median = 527 days, range = 3–2856, IQR = 225–1014). The median lifespan of the TIVAPs was 18 months (range 1–94).
Complications (Table 3)
Eighty-seven complications occurred, i.e., an incidence of 0.237 complications/1000 catheter-days (95% Poisson exact CI, 0.190 to 0.292). The median time to occurrence of any complications was 128 days (range 6 to 2533). Forty-nine (56%) complications occurred within 6 months, 13 (15%) between 6 and 12 months (62 (74.7%) within a year), and only 25 (29%) after 1 year. The types of complication are summarized in Table 4. No complications occurred in the 10 second TIVAPs implanted in 2006.
Thrombotic complications
Seventeen patients (3.7%) developed venous thrombosis in the catheter or catheterized vein representing an incidence of 0.046 thromboses/1000 catheter-days (95% Poisson exact CI 0.027 to 0.074). The median time to the development of thrombotic complication was 172 days (range 25 to 1300). These venous thromboses were not complicated by any case of symptomatic pulmonary embolism. One patient developed suppurative thrombophlebitis treated with anticoagulation and antibiotic therapy.
Infectious complications
Thirty-seven infectious complications were documented, representing an incidence of 0.101/1000 catheter-days (95% Poisson exact CI 0.071 to 0.139) with a median time to development of 128 days (range 6 to 2533). Twelve TIVAP infections occurred, 5 due to Staphylococcus non-aureus, 3 to Staphylococcus aureus, 1 to a Pseudomonas, 1 to a Gram-negative bacillus, 1 to another organism, and 1 infection halted with empirical antibiotic therapy which was started before the samples were taken. Twenty-four cases of septicemia occurred (7 due to Staphylococcus aureus, 8 due to Staphylococcus non-aureus, 4 due to E. coli (including one case of catheter-related suppurative thrombophlebitis), 1 due to Yersinia, 3 due to other germs, and 1 septicemia halted by previous empirical antibiotic therapy).
Other complications
We recorded nine cases of extravasation, none of which involved the irritant substance. There were 18 other complications including 5 catheter separations or ruptures. The TIVAP was removed in 3 cases.
Complication-related deaths
Ten patients died within a month after their complication. Three deaths were attributed by clinicians to the consequences of the complication. These were one case of E.coli TIVAP suppurative thrombophlebitis, one case of Staphylococcus non-aureus septicemia, and one case of post-chemotherapy febrile neutropenia complicated by septic shock due to a Gram-negative bacillus. The other patients died of tumor progression (N = 6) or from an ischemic stroke (N = 1).
Removal of the TIVAP
One hundred and forty-nine TIVAPs were removed, 108 at the end of chemotherapy, and 41 because of complications, including 9 TIVAP infections, 9 cases of septicemia, and 9 cases of venous thrombosis. These results are detailed in Table 5. Median time to removal of the TIVAP because of a complication was 264 days after the implantation (range 8 to 2533).
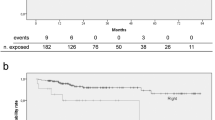
Risk factors for complications
As few thrombotic and infectious complications occurred, it was decided to pull them. The cumulative risk of these complications was low (Fig. 1). The following factors were found to independent prognostic indicators for the development of a thromboembolic and/or infectious complication on multivariate analysis: age >75 years (HR = 2.03, 95% CI = 1.07–3.86, p = 0.031), anticoagulant therapy prior to TIVAP implantation (HR = 1.95, 95% CI = 1.03–3.71. p = 0.040) and implantation of the device on the left side (HR = 1.72, 95% CI = 1.01–2.94, p = 0.046) Table 4.
Discussion
Totally implantable venous access ports have considerably improved the management of patients suffering from cancer by facilitating the administration of chemotherapy, blood transfusions, antibiotics, parenteral nutrition and analgesics, etc. The implantation of these devices, however, is associated with both early complications from the implantation procedure and late complications from their long-term use. There is relatively limited information available about all of the potential complications, and in particular, few prospective studies have examined the entire range of possible complications in a significant number of patients.
We carried out a longitudinal study in a single-center cohort of 493 TIVAPs with a median follow-up of 18 months (1–94), i.e., an experience of 367,359 catheter-days. This follow-up period is much longer than those reported in the literature (6098 to 183,467 catheter-days); only Kock’s study [9] had a slightly larger experience (400,000 catheter-days) (Table 5).
In our study, the overall incidence of complications was 0.237/1000 catheter-days with very few major complications.
The rate of symptomatic thromboembolism complication was 3.7%, i.e., 0.046/1000 catheter-days, which is less than the figure reported by Lee AY et al. [11] of 0.3/1000 catheter-days. No case of pulmonary embolism occurred. The thromboembolic complication rates in other studies have ranged from 0.3 to 28.3%. These rates are higher when patients are investigated routinely by Doppler ultrasonography (27–66%) [3]. However, the clinical meaningfulness of such routine examination has not been evaluated. Symptomatic pulmonary embolism rates range from 15 to 25% [19]. Very surprisingly, we found no cases of catheter occlusion without venous thrombosis, whereas Lee et al. [11] reported 50 cases of catheter occlusion, 12 of which were complicated by deep venous thrombosis. According to Lee AY et al., catheter occlusions are significantly associated with deep vein thrombosis.
The risk factors for thrombotic complications have previously been identified and include vascular trauma (implantation of two or more TIVAPs, several attempts at catheterization, large diameter or multi-lumen catheters), ovarian cancer compared to other cancers, incorrect positioning of the proximal tip of the catheter in the superior vena cava, implantation on the right side, and a past history of chest irradiation [11, 20]. None of these risk factors were confirmed in our series as the thrombotic event rate was too low.
The incidence of infectious complications was low (0.101/1000 catheter-days) and similar to published data (0.15 to 0.39/1000 catheter-days) [21, 22]. The difficulty in this situation is discriminating between primary TIVAP infections and hematogenous infections arising from remote sepsis. Some of the septicemia cases probably originated from a urinary tract infection or gastrointestinal tumor. In the clinician’s opinion, only 13 cases of primary TIVAP infections were seen (i.e., an incidence of 0.035/1000 catheter-days). The documented organisms were consistent with what would be expected, the majority being a coagulase-negative staphylococcus. We found no cases of fungal infection, probably because induction therapy for acute leukemia and peripheral autologous stem cell transplantation are not carried out in our center. The risk factors for infectious complications of a TIVAP have been extensively studied and include the frequency of TIVAP punctures, administration of parenteral nutrition, loss of autonomy, metastatic progression, and young patient age [4]. We were not able to confirm the role of these risk factors due to the small number of infectious events in our series.
Overall, thrombotic and infectious complications were infrequent in our series and were seen principally during the first year after implantation. Repeated punctures of the chamber did not appear to increase the risk of infection if adequate preventive measures were taken.
This was a prospective study carried out under real-life conditions. The organization of the study was optimized in order to exhaustively record all of the events, which occurred during the use of a TIVAP. Our findings indicate that implantation and long-term use of a TIVAP are safe. The small number of complications can be explained by improved materials that are less thrombogenic materials, better operating techniques, and increased training for nurses who handle the TIVAPs.
The low incidence of complications makes it difficult to identify associated risk factors, although this analysis ultimately is of limited benefit as the complications themselves are rare.
In conclusion, the long-term follow-up of a large single-center series of TIVAPs shows that the use of these devices by an experienced team is associated with a low complication rate. Because of the low incidence of these complications, we were unable to identify any new prognostic indicators or confirm those which have already been described. The findings we report can be used to inform patients in whom implantation of a TIVAP is proposed and favor an implantation of TIAVP for the vast majority of patients treated with cytotoxic chemotherapy.
References
Sznajder JL, Zveibil FR, Bitterman H et al (1986) Central vein catheterization: failure and complication rates by three percutaneous approaches. Arch Intern Med 146:259–261
Mansfield PF, Hohn DC, Fornage BD et al (1994) Complications and failures of subclavian-vein catheterization. N Engl J Med 331:1735–1738
Verso M, Agnelli G (2003) Venous thromboembolism associated with long-term use of central venous catheters in cancer patients. J Clin Oncol 21(19):3665–3675
Lebeaux D, Larroque B, Gellen-Dautremer J, Leflon-Guibout V, Dreyer C, Bialek S, Froissart A, Hentic O, Tessier C, Ruimy R, Pelletier AL, Crestani B, Fournier M, Papo T, Barry B, Zarrouk V, Fantin B (2012) Clinical outcome after a totally implantable venous access port-related infection in cancer patients: a prospective study and review of the literature. Medicine 91(6):309–318
Biffi R, Pozzi S, Agazzi A, Pace U, Floridi A, Cenciarelli S, Peveri V, Cocquio A, Andreoni B, Martinelli G (2004) Use of totally implantable central venous access ports for high-dose chemotherapy and peripheral blood stem cell transplantation: results of a monocentre series of 376 patients. Ann Oncol 15:296–300
Bassi KK, Giri AK, Pattanayak M, Abraham SW, Pandey KK (2012) Totally implantable venous access ports: retrospective review of long-term complications in 81 patients. Indian J Cancer 49(1):114–118
Prandoni P, Polistena P, Bernardi E, Cogo A, Casara D, Verlato F, Angelini F, Simioni P, Signorini GP, Benedetti L, Girolami A (1987) Upper-extremity deep vein thrombosis. Risk factors, diagnosis, and complications. Arch Intern Med 157(1):57–62
Hull RD, Hirsh J, Carter CJ, Jay RM, Dodd PE, Ockelford PA, Coates G, Gill GJ, Turpie AG, Doyle DJ, Buller HR, Raskob GE (1983) Pulmonary angiography, ventilation lung scanning, and venography for clinically suspected pulmonary embolism with abnormal perfusion lung scan. Ann Intern Med 98(6):891–899
Kock HJ, Pietsch M, Krause U, Wilke H, Eigler FW (1988) Implantable vascular access systems: experience in 1500 patients with totally implanted central venous port systems. World J Surg 22(1):12–16
Wolosker N, Yazbek G, Nishinari K et al (2004) Totaly implantable venous catheters for chemotherapy: experience in 500 patients. Sao Paulo Med J 122:147–151
Lee AY, Levine MN, Butler G, Webb C, Costantini L, Gu C, Julian JA (2006) Incidence, risk factors, and outcomes of catheter-related thrombosis in adult patients with cancer. J Clin Oncol 24(9):1404–1408
Penel N, Neu J-C, Clisant S et al (2007) Risk factors for early catheter-related infections in cancer patients. Cancer 110:1586–1592
Biffi R, Orsi F, Pozzi S, Pace U, Bonomo G, Monfardini L, Della Vigna P, Rotmensz N, Radice D, Zampino MG, Fazio N, de Braud F, Andreoni B, Goldhirsch A (2009) Best choice of central venous insertion site for the prevention of catheter-related complications in adult patients who need cancer therapy: a randomized trial. Ann Oncol 20(5):935–940
Nishinari K, Wolosker N, Bernardi CV, Yazbek G (2010) Totally implantable ports connected to valved catheters for chemotherapy: experience from 350 Groshong devices. J Vasc Access 11(1):17–22
Heibl C, Trommet V, Burgstaller S, Mayrbaeurl B, Baldinger C, Koplmüller R, Kühr T, Wimmer L, Thaler J (2010) Complications associated with the use of Port-a-Caths in patients with malignant or haematological disease: a single-centre prospective analysis. Eur J Cancer Care 19(5):676–81
Revel-Vilk S, Yacobovich J, Tamary H et al (2010) Risk factors for central venous catheter thrombotic complications in children and adolescents with cancer. Cancer 116:4197–4205
Piran S, Ngo V, McDiarmid S et al (2014) Incidence and risk factors of symptomatic venous thromboembolism related to implanted ports in cancer patients. Thromb Res 133:30–33
Joks M, Czyz A, Poplawski D, Komarnicki M (2014) Incidence and risk factors for centrral venous catheter-related thrombosis in hematological patients. Med Oncol 31(1):772
Agnelli G, Verso M (2006) Therapy Insight: venous-catheter-related thrombosis in cancer patients. Nat Clin Pract Oncol 3(4):214–222
Verso M, Agnelli G, Kamphuisen PW, Ageno W, Bazzan M, Lazzaro A, Paoletti F, Paciaroni M, Mosca S, Bertoglio S (2008) Risk factors for upper limb deep vein thrombosis associated with the use of central vein catheter in cancer patients. Intern Emerg Med 3(2):117–122
Groeger JS, Lucas AB, Thaler HT, Friedlander-Klar H, Brown AE, Kiehn TE, Armstrong D (1993) Infectious morbidity associated with long-term use of venous access devices in patients with cancer. Ann Intern Med 119(12):1168–1174
Chang L, Tsai JS, Huang SJ, Shih CC (2003) Evaluation of infectious complications of the implantable venous access system in a general oncologic population. Am J Infect Control 31(1):34–39
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The research has not been sponsored. The authors declare that they have no conflict of interest.
We have full control of all primary data and allow the journal to review all the data.
Rights and permissions
About this article
Cite this article
Voog, E., Campion, L., du Rusquec, P. et al. Totally implantable venous access ports: a prospective long-term study of early and late complications in adult patients with cancer. Support Care Cancer 26, 81–89 (2018). https://doi.org/10.1007/s00520-017-3816-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-017-3816-3