Abstract
Introduction
Laryngeal nerve monitoring has been increasingly embraced as a mechanism for mitigating the risk of nerve damage during thyroid and parathyroid surgery. Vagal nerve monitoring has recently been introduced as a potentially increased level of nerve integrity scrutiny. We sought to define the risks and benefits of this technology in a prospective analysis of a series of patients undergoing neck endocrine surgery.
Setting
High-volume academic endocrine surgery practice.
Methods
A prospective, non-controlled trial of continuous vagal nerve monitoring (CVNM) in a projected cohort of 20 non-randomly selected patients undergoing thyroid and parathyroid surgery was planned. A commercially available nerve monitoring system with automatic periodic stimulation was utilized for both laryngeal nerve monitoring and CVNM. Demographic data were obtained, and outcome variables included surgical procedures performed, pathology, complications, incremental time required to achieve CVNM, and benefits of monitoring and stimulation.
Results
The patient accrual was aborted after 9 surgeries (12 nerves monitored) because of two serious adverse events (hemodynamic instability and reversible vagal neuropraxia attributable to the monitoring apparatus). No other complications occurred. The time to establish monitoring ranged from 3 to 26 min, with a median of 6 min (representing 2.9–12.2 % of the total surgical procedural time). The stimulation clamp became dislodged 11 times in 5 cases and was replaced in 7 of those instances. Benefits of CVNM included recognition of reduced amplitude and increased nerve latency in two patients.
Conclusions
We report the first evidence that CVNM may cause serious patient harm. This novel approach is invasive and threatens patient safety. Although it may occasionally provide meaningful information, the risk–benefit ratio does not favor widespread adoption.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroid surgery was transformed by Theodore Kocher in the late nineteenth century from an undertaking famously labeled “horrid butchery” [1] to a procedure with low mortality and acceptable morbidity. Nevertheless, RLN injuries continued to occur at a high rate.
A second series of incremental technical advances in the past three decades allowed the thyroidectomy procedure to continue to evolve. These improvements related largely to improved anatomical understanding; the old adage “a nerve seen is a nerve injured” [2] gave way to an appreciation that nerve identification is superior to nerve avoidance [3].
More recently, the concept of nerve monitoring has gained traction. Through the influence of proponents such as Randolph et al. [4] and Snyder and Hendricks [5] and the comprehensive and meticulous contributions of Dralle et al., [6] widespread implementation across geographic areas and surgical disciplines has occurred. In particular, the capability of stimulating the first dissected nerve after a lobectomy has been completed in order to derive predictive physiologic information regarding nerve function allows a more informed and perhaps altered dissection of the contralateral lobe. Introduction of user-friendly devices and interfaces and improved reliability were critical to promoting large scale implementation of nerve monitoring. A number of investigators have advocated for routine stimulation of the vagus nerve as part of the nerve monitoring algorithm [4]. More recently, a device has been designed to allow for repetitive and nearly continuous stimulation of the vagus nerve throughout the thyroidectomy procedure [7]. We systematically assessed this new technology in a prospective manner.
Materials and methods
A prospective, non-controlled trial of continuous vagal nerve monitoring (CVNM) in a projected 20-patient cohort was planned. A non-randomized population was selected from a group of individuals undergoing thyroid or parathyroid surgery at the Georgia Regents University Thyroid and Parathyroid Center between 2/26/2014 and 6/25/2014 based on the clinical judgment of the senior author of the likelihood of benefit from a higher level of nerve integrity scrutiny. These included patients with large and substernal goiters, surgery on an only functioning nerve, reoperative surgery, and known cancer. Institutional review board approval was sought and granted to analyze data from a prospectively maintained quality assurance database (Approval # Pro00000155).
Vagal nerve stimulation
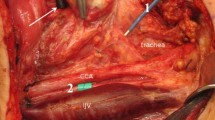
The NIM® 3.0 Nerve Integrity Monitoring System and the APS Electrode (Automatic periodic stimulation, Medtronic, Minneapolis, MN) were utilized for laryngeal nerve monitoring and repetitive stimulation of the vagus nerve (Fig. 1).
Electrode placement
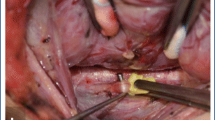
The preliminary steps of thyroidectomy and parathyroidectomy were performed, including separation of the strap muscles in the midline, elevation of the strap muscles off of the ventral surface of the thyroid gland and mobilization of the lateral and posterior surfaces of the thyroid gland. The carotid sheath was then exposed using one of three approaches (adjacent to the thyroid, between the sternohyoid and sternothyroid muscles, and lateral to the sternohyoid and sternothyroid muscles) to determine the optimal positioning of the electrode. The vagus nerve was identified by blunt dissection within the carotid sheath and carefully dissected circumferentially for a short segment. The APS stimulating electrode was placed on this dissected segment of the vagus nerve, with attention to ensure that the nerve was completely encircled (Fig. 2). Once the electrode was in place, baseline measurements of amplitude and nerve latency were acquired with a stimulation intensity of 1.0 mA. Dissection of the thyroid or parathyroid gland(s) was then continued during which the electrode delivered intermittent stimulation to the vagus nerve, programmed to occur every 6 s at a stimulation intensity of 1.0 mA. Alert thresholds for reduced signal amplitude and increased nerve latency were set according to the manufacturer recommendations (greater than 50 % decrease in signal amplitude or 10 % increase in signal latency triggers an alarm). Electromyographic responses of the laryngeal muscles were displayed in real time.
Data acquisition
The time from initiation of dissection of the vagus nerve to the placement of APS electrode around the nerve was recorded. In cases where the electrode was inadvertently dislodged during the case, the time to replace the electrode was also recorded.
Vagal evoked waveform reductions in amplitude and increases in latency were noted. A determination was made if the event trigger was consistent with the concomitant surgical activity; additionally, the recurrent laryngeal nerve was stimulated with a monopolar probe at the time of the event trigger to verify continuing signal. This stimulation was repeated at the completion of dissection.
Preoperative and immediate postoperative flexible fiberoptic laryngoscopy was performed in all patients who were uniformly managed on an outpatient basis according to longstanding protocol [8].
Results
Twelve nerves were monitored in a total of nine patients who underwent CVNM during their thyroid and parathyroid surgeries. Of these nine surgeries, four were reoperative procedures. Incision lengths ranged from 3 to 12 cm. Six cases revealed papillary thyroid carcinoma and three revealed benign pathology. A 2-mm APS electrode was utilized in six nerves, and a 3-mm electrode was utilized in another six nerves (the 2- and 3-mm electrodes were chosen at random) (Table 1).
The mean and median times required for initial placement of the APS electrode were 7.9 ± 6.1 and 6 min, respectively. The longest time to achieve electrode placement was 26 min due to significant scar tissue encountered during the dissection for completion thyroidectomy. The stimulation clamp became dislodged (usually from inadvertent tugging on the wire which courses within the operative field) 11 times in 5 cases and was replaced in 7 of those instances. The rate of dislodgement was similar between the 2 and 3 mm APS electrodes. The mean and median times recorded for replacement of the electrode were 2.3 ± 1.3 and 2 min, respectively. The proportion of overall operative time required for placement of the APS electrodes range from 2.9 to 12.2 %, with a mean of 7.3 ± 3.4 %.
An event trigger notifying the surgeons of decreased amplitude and increased latency from baseline nerve function occurred three times during the study period. The event correlated with retraction on the thyroid gland in one case (and normal amplitude and latency were restored with release of retraction). In the second case, increased traction on the RLN was recognized during dissection; similarly, normal waveforms were restored after reduced traction. In both the cases, the RLN stimulated normally at 1.0 mA at the completion of dissection, and the true vocal folds were mobile bilaterally on postoperative laryngoscopy (Table 2). In the final instance, the RLN was found to be encased in tumor. During dissection, an APS event trigger corresponded with loss of ability to stimulate the RLN at 1.0 mA. Because of the presence of distant metastasis, a decision was made to not resect the nerve. The corresponding vocal fold was found to be hypomobile on flexible laryngoscopy in the post-anesthesia care unit. The nerve dysfunction resolved 1 month postoperatively.
Two serious complications occurred as a direct result of the use of CVNM (Table 3). In the first complication, the APS electrode was inadvertently and traumatically dislodged, causing vagal neuropraxia. Visible perineural ecchymosis of the vagus nerve could be appreciated. Immediate attempts to stimulate both the vagus nerve and the RLN were unsuccessful. The corresponding vocal fold was found to be hypomobile on flexible laryngoscopy in the post-anesthesia care unit. The nerve dysfunction resolved 1 month postoperatively.
The most serious complication that was observed was a case of hemodynamic instability, manifested as bradycardia and hypotension, which occurred shortly after baseline calibration of the 2-mm APS electrode in a young (33-year old) healthy woman with no cardiac history. Hemodynamic stability was promptly reestablished when the electrode was removed. A second attempt to establish CVNM was undertaken. The APS electrode was replaced and was followed quickly by an identical hemodynamic response, which again reversed promptly when the electrode was removed (Fig. 3). The vagal nerve monitoring was therefore abandoned. Of note, there were no hemodynamic abnormalities during the process of identification and dissection of the vagus nerve, and no other episodes of hemodynamic instability were observed for the remainder of the surgery.
Discussion
After considerable modification, thyroid surgery has become a procedure that can be accomplished with an exceptionally low mortality rate. Better understanding of the relevant anatomy has substantially improved the complication profile as well. This has naturally prompted attention to turn to further technical refinements, including better or non-visible scars, [9] streamlined care (including routine outpatient management [8]), and enhanced detection of thyroid conditions with rational and thoughtful implementation of surgery [10]. Despite this progress, the two principal complications of hypoparathyroidism and nerve dysfunction persist even in the most experienced hands.
While there have been recent promising developments in the ability to intraoperatively identify parathyroid tissue, thereby offering the promise of minimizing damage, [11, 12] most of the attention has been directed toward improving the ability to prevent nerve injury. The early attempts to accomplish nerve monitoring were crude and somewhat invasive, including implantation of hook-wire electrodes into the vocal cords prior to undertaking thyroid surgery [13]. It was quickly recognized that this invasive modality would not serve as an acceptable solution, and investigation into the feasibility of surface electrodes was pursued [14]. This technology has steadily improved, with a number of excellent options now available. Vagal nerve stimulation as part of the nerve monitoring pathway is logical and has been promoted in a number of publications [4, 7, 15] but would seem not to be necessary on a routine basis, particularly if unilateral surgery is being performed. Even as debate continues on the value of post-recurrent laryngeal nerve dissection vagal nerve stimulation, there has been industry-led promotion of an even more invasive technique consisting of implanting a stimulating device circumferentially around the vagus nerve to facilitate more frequent stimulation. This has evoked concerns about the technology representing the proverbial “too much of a good thing,” and our group was motivated to carefully assess this technological opportunity. Although the term “continuous vagal nerve monitoring (CVNM)” has emerged as the common parlance to describe this new technique [16] (and is therefore utilized here), it is somewhat misleading and implies a process different from automatic periodic stimulation (which is simply repetitive, intermittent stimulation).
We systematically assessed the use of this automatic periodic stimulation technology in a series of selected patients. We aborted our assessment because of a serious adverse event (cardiac arrhythmia). This complication relates to the increased parasympathetic tone caused by the vagal stimulation, as was described previously by Friedrich and co-authors in a small series of five patients [17]. Even if we had accrued the intended number of patients, the overall unacceptable increase in duration of surgery and introduction of new potential complications to the thyroidectomy procedure, with limited associated benefit, would have led to a conclusion that this technology is not worth the risk. As can be seen from our data, incorporation of this additional mechanism of nerve monitoring required on average 7.9 min to establish per nerve monitored. For bilateral surgery, this would equate to an additional 16 min of surgery. While additional information was occasionally derived from this monitoring, it is unlikely that this information would have materially changed the outcome in any of the nine patients. Furthermore, while with recurrent laryngeal nerve monitoring, an ongoing controversy exists regarding the cost-benefit ratio of this modality, with automatic period stimulation of the vagus nerve, we are forced to consider a risk–benefit ratio.
Much like axillary thyroidectomy, where new complications not previously associated with thyroid surgery were introduced [18], automatic periodic stimulation of the vagus nerve has introduced the very real potential for jugular vein, carotid artery, and vagus nerve injury, as well as the potential for cardiac arrhythmias, as was experienced in one of our nine patients. While the importance of both anatomic and physiologic preservation of recurrent laryngeal nerve function cannot be overstated, we also are obligated not to harm our patients in an effort to reduce a complication rate that does not occur in 99 % of patients. Although it may occasionally provide meaningful information, we judged the risk–benefit ratio of CVNM to be skewed heavily toward unacceptable additional risk of harm [19, 20] and therefore embargoed further utilization of this invasive technology in our patients.
We acknowledge that automatic periodic stimulation of the vagus nerve may represent a useful technology for other surgeries where the RLN is at risk [21]. In those operations where it is inconvenient to identify and stimulate a vulnerable RLN, it is possible that CVNM is preferable.
We conclude that, while there are theoretical advantages to implementation of repetitive vagal nerve stimulation as part of a nerve monitoring algorithm in thyroid surgery, the approach, at least in its current form, not only fails to represent an advance for thyroid surgeons but it may also in fact constitute a step backward from the very safety that we are trying to achieve.
References
Holm TM, Pai SI (2013) The superior laryngeal nerve. In: Miccoli P, Terris DJ, Minuto MN, Seybt MW (eds) Thyroid surgery: preventing and managing complications. Wiley-Blackwell, Oxford
Prioleau WH (1933) Injury of laryngeal branches of vagus nerve in thyroid surgery. South Surg 1:287–292
Lahey FH, Hoover WB (1938) Injuries to the recurrent laryngeal nerve in thyroid operations: their management and avoidance. Ann Surg 108(4):545–562
Randolph GW, Dralle H, International Intraoperative Monitoring Study Group (2011) Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope 121(Suppl 1):S1–S16
Snyder SK, Hendricks JC (2005) Intraoperative neurophysiology testing of the recurrent laryngeal nerve: plaudits and pitfalls. Surgery 138(6):1183–1191
Dralle H, Sekulla C, Haerting J, Timmermann W, Neumann HJ, Kruse E, Grond S, Mühlig HP, Richter C, Voss J, Thomusch O, Lippert H, Gastinger I, Brauckhoff M, Gimm O (2004) Risk factors of paralysis and functional outcome after recurrent laryngeal nerve monitoring in thyroid surgery. Surgery 136(6):1310–1322
Schneider R, Randolph GW, Sekulla C, Phelan E, Thanh PN, Bucher M, Machens A, Dralle H, Lorenz K (2013) Continuous intraoperative vagus nerve stimulation for identification of imminent recurrent laryngeal nerve injury. Head Neck 35(11):1591–1598
Seybt MW, Terris DJ (2010) Outpatient thyroidectomy: experience in over 200 patients. Laryngoscope 120(5):959–963
Miccoli P, Berti P, Materazzi G, Massi M, Picone A, Minuto MN (2004) Results of video-assisted parathyroidectomy: single institution’s six-year experience. World J Surg 28(12):1216–1218. doi:10.1007/s00268-004-7638-3
Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM, American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer (2009) Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19(11):1167–1214
McWade MA, Paras C, White LM, Phay JE, Mahadevan-Jansen A, Broome JT (2013) A novel optical approach to intraoperative detection of parathyroid glands. Surgery 154(6):1371–1377
James BC, Nagar S, Tracy M, Kaplan EL, Angelos P, Scherberg NH, Grogan RH (2014) A novel, ultrarapid parathyroid hormone assay to distinguish parathyroid from nonparathyroid tissue. Surgery 156(6):1638–1643
Yarbrough DE, Thompson GB, Kasperbauer JL, Harper CM, Grant CS (2004) Intraoperative electromyographic monitoring of the recurrent laryngeal nerve in reoperative thyroid and parathyroid surgery. Surgery 136(6):1107–1115
Eisele DW (1996) Intraoperative electrophysiologic monitoring of the recurrent laryngeal nerve. Laryngoscope 106(4):443–449
Wu CW, Dionigi G, Chen HC, Chen HY, Lee KW, Lu IC, Chang PY, Hsiao PJ, Ho KY, Chiang FY (2013) Vagal nerve stimulation without dissecting the carotid sheath during intraoperative neuromonitoring of the recurrent laryngeal nerve in thyroid surgery. Head Neck 35(10):1443–1447
Phelan E, Schneider R, Lorenz K, Dralle H, Kamani D, Potenza A, Sritharan N, Shin J, Randolph GW (2014) Continuous vagal IONM prevents recurrent laryngeal nerve paralysis by revealing initial EMG changes of impending neuropraxic injury: a prospective, multicenter study. Laryngoscope 124(6):1498–1505
Ulmer C, Friedrich C, Kohler A, Rieber F, Basar T, Deuschle M, Thon KP, Lamadé W (2011) Impact of continuous intraoperative neuromonitoring on autonomic nervous system during thyroid surgery. Head Neck 33(7):976–984
Kuppersmith RB, Holsinger FC (2011) Robotic thyroid surgery: an initial experience with North American patients. Laryngoscope 121(3):521–526
McCulloch P et al (2009) No surgical innovation without evaluation: the IDEAL recommendations. Lancet 374(9695):1105–1112
Angelos P (2010) The ethical challenges of surgical innovation for patient care. Lancet 376(9746):1046–1047
Hemmerling TM (2008) Monitoring neural function during surgery around the glossopharyngeal, vagus and laryngeal nerves during neck (thyroid, larynx, carotid) and chest procedures. Handbook of clinical neurophysiology, vol. 8. pp 590–606
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Terris, D.J., Chaung, K. & Duke, W.S. Continuous Vagal Nerve Monitoring is Dangerous and Should not Routinely be Done During Thyroid Surgery. World J Surg 39, 2471–2476 (2015). https://doi.org/10.1007/s00268-015-3139-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-015-3139-9