Abstract
Background
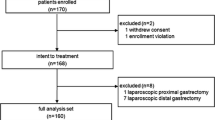
This retrospective study was designed to assess the feasibility of laparoscopic total gastrectomy (LTG) in clinical stage I gastric cancer patients, and validate the appropriateness of the widespread adoption of LTG for experienced open surgeons.
Methods
Eighty-eight patients with clinical stage I gastric cancer underwent LTG in our hospitals (n = 55) and affiliated hospitals (n = 33). Esophagojejunostomy was performed intracoporeally using a circular stapler with an incision in the left upper abdomen. We investigated the patients’ clinicopathologic factor, and evaluated the effect of hospital volume on short-term outcomes.
Results
Fixed insertion of the anvil head was successfully achieved in all patients (lift-up method in 58 patients and transoral method in 28 patients), although 2 patients were converted to open surgery. The approach using a circular stapler through a small incision from the upper left quadrant of the abdomen facilitated a good laparoscopic visual field for the plane of the esophagojejunostomy. Fourteen patients developed Clavien–Dindo classification grade II or more postoperative complications, and the overall operative morbidity rate was 15.9 %. No anastomotic leakage was encountered in this series. No significant difference was observed in clinical outcomes between patients in the high- and low-volume hospital groups.
Conclusions
Laparoscopic total gastrectomy can be performed safely on clinical stage I gastric cancer patients by surgeons with sufficient experience in open gastrectomy and therefore represents a feasible procedure that is not clinically impacted by hospital volume.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laparoscopic gastrectomy has recently been widely performed because it is less invasive than traditional surgical techniques [1–4]. Laparoscopic total gastrectomy (LTG), however, is not yet widespread compared to laparoscopic distal gastrectomy (LDG). Although expert laparoscopic surgeons have reported the safety and feasibility of LTG in a limited numbers of institutions [5–8], many surgeons still hesitate to perform LTG, especially esophagojejunostomy, owing to technical complexities and difficulties.
The fundamental difference between LDG and LTG is the difficulty of reconstruction. Reconstruction has often been performed through mini-laparotomy in LTG; therefore, esophagojejunostomy, which consists of fixation of the anvil head and insertion of a circular stapler into the abdominal cavity, was performed through an upper median mini-laparotomy when LTG was first being developed. However, a good view of the esophageal stump is difficult to be secured during esophagojejunostomy, sometimes resulting in anastomotic complications. More recently, several novel laparoscopic procedures for anvil fixation into the esophageal stump have been developed and shown to contribute to safe anastomosis [9–11]. These reports of these procedures, however, were based on the results of LTG performed by experienced laparoscopic surgeons in a small number of hospitals.
This retrospective study was designed to assess the feasibility and surgical outcomes of LTG in clinical stage I gastric cancer patients and to evaluate the appropriateness of the widespread adoption of LTG for experienced open surgeons, as well as the effect of hospital volume on short-term patient-outcomes.
Materials and methods
Patients
Eighty-eight patients with clinical stage I gastric cancer (T1N0, T1N1, and T2N0) underwent LTG with D1 plus or D2 lymph node dissection between 2007 and 2013 at Kyoto Prefectural University of Medicine and affiliated hospitals (10 hospitals listed in the Acknowledgements section). The indication for LTG consisted of a tumor located in the upper- and/or middle-third of the stomach. Preoperative clinical diagnoses of tumor invasion and metastasis were generally made based on the findings of endoscopy, barium study, and computed tomography (CT). Written informed consent was obtained from all patients prior to surgery. Patients were converted to open surgery if there were intraoperative findings of more advanced disease, such as serosal invasion and/or extended nodal metastases. The clinicopathological factors and postoperative complications of these patients were reviewed retrospectively from hospital records. The macroscopic and microscopic classification of gastric cancer was based on the Japanese Classification of Gastric Carcinoma [12].
The annual average number of gastrectomies was over 100 at our university hospital, whereas the average numbers of gastrectomies were less than 30 at most of the affiliated hospitals. After the first author (D.I.) performed the procedure on 30 cases as an operating surgeon, LTG was also performed by three surgeons (S.K., T.K., K.O.) under the supervision of D.I. in our hospital. On the other hand, LTG was performed by surgeons at affiliated hospitals at their request. In the affiliated hospitals, doctors who had performed open gastrectomy on at least 30 patients performed LTG, while D.I. guided the operation as an assistant. The procedure for the first 30 cases in our university hospital was also the same as that described below; however, the indication was cancer clinically diagnosed as T1N0 only.
Surgical procedure for LTG
With the patient under general anesthesia, a 12-mm port was inserted through the umbilicus using the open technique. After pneumoperitoneum with carbon dioxide, five trocars were inserted: one below the xiphoid appendix for liver retraction, and the remaining four at the bilateral hypochondrium and the mid-abdomen.
From the left side of the patient, the greater omentum was divided 3 cm from the right gastroepiploic vessels using laparoscopic coagulating shears (LCS) (Harmonic Scalpel, Ethicon Endo-Surgery, Cincinnati, OH, USA). The right gastroepiploic vessels were clipped and divided. From the right side of the patient, the left side of the greater omentum was divided toward the lower pole of the spleen, and the left gastroepiploic and short gastric vessels were divided. After retracting the left lobe of the liver using a snake retractor (Stryker, San Jose, CA, USA), the right gastric vessels were clipped and divided. The gastro-pancreatic ligament was then lifted toward the ventral side with the assistant’s right hand grasper, and the supra-pancreatic lymph nodes were dissected. The left gastric vein was then exposed and dissected. The left gastric artery was exposed from the left side, double clipped, and divided. The posterior and short gastric vessels were divided, and the regional lymph nodes (right and left cardial, along the short gastric arteries) were also dissected. Lymph node dissections were performed based on the Guidelines of the Japanese Gastric Cancer Association.
The anvil of a circular stapler was placed into the esophageal stump using the “lift-up” anvil insertion method, which was previously reported by Hiki et al. (Fig. 1.) [9] or using a transorally inserted anvil method (Orvil™, Covidien, Mansfield, MA, USA) [13]. The transoraly inserted anvil method was only used in patients with tumors in which the oral margin was approximately less than 2 cm from the esophagogastric junction. The left upper trocar incision was extended to 4 cm, and the entire stomach, with the regional lymph nodes, was removed through the incision after protection of the wound using a wound-sealing device (Wound Protector and Retractor, Applied Medical, Rancho Santa Margarita, CA, USA).
Intraoperative view of the “lift-up” method. a The anterior wall of the abdominal esophagus was incised, and nasogastric tube was pulled out. b–d The anvil connected to the nasogastric tube was pulled up into the esophageal lumen. e After the anvil insertion, transection of the esophagus was performed using a linear stapler to keep the insertion opening as tight as possible. f This approach from the left side facilitated a good laparoscopic visual field, and prevented the surrounding fatty tissues and organs from intervening between the anastomosis planes
The jejunum, 30 cm from the ligament of Treitz, was marked and removed from the abdominal cavity through the small incision and transected with a linear stapler, followed by the jejunojejunal anastomosis in a side-to-side fashion using the linear stapler. The length between the esophagojejunostomy and the jejunojejunostomy was approximately 40 cm. The head of a circular stapler was introduced into the distal segment of the transected jejunum, passed through the small incision into the abdominal cavity, and guided in the direction of the esophageal stump through the antecolic route from the left side abdominal wall. The opening through which the circular stapler was inserted was then closed using a linear stapler. The Petersen and jejunojejunostomy mesenteric defects were closed with interrupted sutures.
Postoperative complications
Postoperative complications were classified by the Clavien–Dindo classification, and recorded cases more than Grade II. Early complications were defined as having occurred within 30 days of surgery, while complications occurring more than 30 days after surgery were defined as late complications. Anastomosis leakage was confirmed by both clinical symptoms and a radiological study with an orally administrated contrast agent. Anastomotic stenosis was defined as difficulties with scope passage, requiring expansion by an endoscopic balloon bougie. Pancreatic fistula was diagnosed comprehensively not only according to drain amylase levels, but also changes in the properties of the drain, clinical findings, laboratory data, and imaging findings, such as ultrasonogrphy (USTG) and/or computed tomography (CT), as previously reported [14]. For example, patients whose drains were removed that developed postoperative fever, leukocytosis, and the collection of peripancreatic fluid detected on USTG and/or CT were also diagnosed with pancreatic fistula.
Statistical analysis
Student’s t test and the Mann–Whitney U test were used to compare continuous variables, and the Chi-square test or Fisher’s exact test was used for categorical variables. Significant differences were accepted at p < 0.05.
Results
Characteristics of patients and tumors
The clinicopathological features of the patients are shown in Table 1. The mean age was 67 years (range 35–89), and the mean body mass index (BMI) was 24 (range 14.9–42.3). The median surgical duration and amount of blood loss were 414 min (range 245–653) and 93 ml (range 0–780), respectively. In total, 71 patients were diagnosed pathologically as stage I (81 %), and 10 and 7 patients were diagnosed pathologically as stage II and III, respectively.
The mean and median numbers of LTG were 3.3 and 3 cases at low-volume hospitals, respectively (range 1–8 cases). The mean and median numbers of LDG performed by surgeons at low-volume hospitals before they started to perform LTG were 7.9 and 7 cases, respectively (range 3–14 cases).
Perioperative findings and postoperative course
The lift-up method was utilized for insertion of the anvil into the esophageal stump in 58 patients, whereas a transoral method (Orvil™) was used for 28 patients. Fixed insertion of the anvil head was successfully achieved in all patients, although 2 patients had to be converted to open surgery because of an unanticipated hemorrhage during the introductory period. The approach using a circular stapler through the mini-laparotomy from the upper left quadrant of the abdomen facilitated a good laparoscopic visual field for the plane of the esophagojejunostomy (Fig. 1.), and no reconstruction-related intraoperative complication was encountered. Postoperative complications are listed in Table 2. Fourteen patients developed Clavien–Dindo classification grade II or more postoperative complications, and the overall operative morbidity rate was 15.9 %. No anastomotic leakage was encountered in this study. One patient had blind loop syndrome and another 2 patients with internal hernia required reoperation. Totally, 6 patients (6.8 %) suffered from major complications (grade IIIa or more). However, no significant difference was observed in complication rates between high- and low-volume hospitals.
Comparison of the clinical course between high- and low-volume hospitals
In the comparison between high- and low-volume hospitals, the body mass index (BMI) values were higher in patients in the high-volume hospital, although no significant differences between the two groups were observed regarding patient age, the number of lymph nodes retrieved, the operation time, or blood loss (Table 1). All LTG procedures in low-volume centers were performed by doctors at the affiliated hospitals and succeeded without severe intraoperative anastomosis-related complications. During the postoperative course, there was no significant difference between the two groups (Table 2).
Discussion
Laparoscopic gastrectomy has rapidly gained popularity since the first report by Kitano et al. [15]. With regard to LDG, several recent reports have shown that this procedure provides not only favorable short-term outcomes but also equivalent oncologic outcomes [1, 2]. Although almost all of the reports are retrospective and inconclusive, LDG has gained wide acceptance as a treatment option for early gastric cancers.
Meanwhile, LTG is still recognized as a difficult procedure because of the technical complexity of the reconstruction and the challenging maneuver of dissecting the distal splenic artery and the splenic hilar lymph nodes. Despite these technical difficulties, several groups recently reported that LTG is feasible and safe using standardized techniques and new laparoscopic instruments [5–8].
Concerning the reconstruction procedure, a variety of surgical techniques have been developed for the establishment of esophagojejunostomy. For instance, some experienced laparoscopic surgeons have developed linear-stapled side-to-side esophagojejunostomy [16–19]. Linear-stapled esophagojejunostomy has the advantages of accessibility through a trocar, operating in a limited working space, and a better visual field. This technique, however, suffers from the disadvantages of creating sufficient length of esophageal stump for anastomosis and the need for hand-suturing skills, especially in the “overlap” method. By contrast, most surgeons perform circular-stapled esophagojejunostomy because they are familiar with this technique in conventional open total gastrectomy [9–11]. Circular-stapled esophagojejunostomy has the advantages of rapid anastomosis and reliable re-establishment of esophagojejunal continuity. However, this type of anastomosis suffers from some technical issues, primarily how to insert and fix the anvil into the esophageal stump. Hiki et al. developed a novel insertion technique for inserting the anvil head into the esophageal lumen [9]; this lift-up method allows easy and safe insertion of the anvil head into the esophageal stump without any skilled techniques, and simultaneous pushing of the tail and pulling of the anvil head via the nasogastric tube can reduce the need for excessive force. After the anvil has been inserted, transection of esophagus maintains the insertion opening as tightly as possible, and pulling down the anvil head to the esophageal stump safely secures the fixation of the anvil head [20]. This method is a double-stapling technique, however anastomotic stricture can be prevented by closing the transverse incision in a longitudinal direction. Recently, a transorally inserted anvil (Orvil™) was introduced as an alternative method for esophagojejunostomy reconstruction [13]. This method is relatively simple and does not require a learning period for surgeons and has been reported to be technically feasible with an acceptable rate of anastomosis-related complications. However, there remains the risk of esophageal injury using the transoral Orvil™ system; therefore, our initial choice for anvil insertion was the lift-up method. The Orvil™ system was used only in patients with tumors in which the oral margin was approximately less than 2 cm from the esophagogastric junction.
In the circular-stapled anastomosis, the circular stapler itself sometimes blocks the visual fields of the anastomotic site because of the limited working space, which may result in inaccurate anastomosis. For this reason, we employed an approach of circular stapling from a small upper left abdominal incision [21]. This approach provided a good visual field of the anastomosis site without unnecessary tension or an awkward angle for the laparoscope, even for obese patients. Moreover, the good laparoscopic view at the site of anastomosis prevented the surrounding fatty tissues and organs from intervening between the anastomosis planes and consequently allowed accurate and safe anastomosis. Our method, which involved the approach of circular stapler from the small upper left abdominal incision, facilitates LTG, at least reconstruction, even for less experienced laparoscopic surgeons. In fact, there were no severe anastomosis-related complications, except for 3 patients with delayed anastomotic stenosis, and no patients required reoperation for anastomosis-related complications in this series. We found 2 patients with Petersen’s hernia in an early phase of the study. Thereafter, all mesenteric defects were closed using interrupted sutures, and then no additional cases of internal hernia were observed. It is not possible to directly compare our results to previous data because of the different publication dates, among other factors. The complication rates, however, were acceptable in our series and were not correlated with the hospital volume. In low-volume hospitals, the LTG procedures were performed by less experienced laparoscopic surgeons who had sufficient experience performing open total gastrectomy. There has been no clear evidence concerning the experience of the surgeons to indicate the number of open gastrectomies or laparoscopic distal gastrectomies that is considered sufficient as experience for LTG. However, we previously reported that surgeons with experience of 30 cases of open gastrectomy could safely perform LDG [22]; therefore, we defined surgeons with experience of 30 cases of open gastrectomy as experienced open surgeons, and they performed LTG in this study. However, there was no significant difference in the number of lymph nodes retrieved between the high- and low-volume groups, which indicate that adequate lymph node dissection was also performed by doctors at the low-volume hospitals. These findings indicate that LTG followed by esophagojejunostomy as described above is a safe and feasible procedure.
Conclusions
Laparoscopic total gastrectomy can be performed safely by surgeons with sufficient experience in open gastrectomy and therefore represents a feasible procedure for clinical stage I gastric cancer patients that is not affected by hospital volume.
References
Huscher CG, Mingoli A, Sgarzini G et al (2005) Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg 241:232–237
Kitano S, Shiraishi N, Uyama I et al (2007) A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg 245:68–72
Katai H, Sasako M, Fukuda H et al (2010) Safety and feasibility of laparoscopy-assisted distal gastrectomy with suprapancreatic nodal dissection for clinical stage I gastric cancer: a multicenter phase II trial (JCOG 0703). Gastric Cancer 13:238–244
Yoshikawa T, Cho H, Rino Y et al (2013) A prospective feasibility and safety study of laparoscopy-assisted distal gastrectomy for clinical stage I gastric cancer initiated by surgeons with much experience of open gastrectomy and laparoscopic surgery. Gastric Cancer 16:126–132
Chen K, Xu XW, Zhang RC et al (2013) Systematic review and meta-analysis of laparoscopy-assisted and open total gastrectomy for gastric cancer. World J Gastroenterol 19:5365–5376
Lee MS, Lee JH, Park do J et al (2013) Comparison of short- and long-term outcomes of laparoscopic-assisted total gastrectomy and open total gastrectomy in gastric cancer patients. Surg Endosc 27:2598–2605
Shim JH, Oh SI, Yoo HM et al (2013) Short-term outcomes of laparoscopic versus open total gastrectomy: a matched-cohort study. Am J Surg 206:346–351
Jeong O, Ryu SY, Choi WY et al (2014) Risk factors and learning curve associated with postoperative morbidity of laparoscopic total gastrectomy for gastric carcinoma. Ann Surg Oncol. doi:10.1245/s10434-014-3666-x
Hiki N, Fukunaga T, Yamaguchi T et al (2007) Laparoscopic esophagogastric circular stapled anastomosis: a modified technique to protect the esophagus. Gastric Cancer 10:181–186
Kinoshita T, Oshiro T, Ito K et al (2010) Intracorporeal circular-stapled esophagojejunostomy using hand-sewn purse-string suture after laparoscopic total gastrectomy. Surg Endosc 24:2908–2912
Wada N, Kurokawa Y, Takiguchi S et al (2014) Feasibility of laparoscopy-assisted total gastrectomy in patients with clinical stage I gastric cancer. Gastric Cancer 17:137–140
Japanese Gastric Cancer Association (2011) Japanese Gastric Cancer Treatment Guidelines 2010 (ver. 3). Gastric Cancer 14:113–123
Ito H, Inoue H, Odaka N et al (2014) Evaluation of the safety and efficacy of esophagojejunostomy after totally laparoscopic total gastrectomy using a trans-orally inserted anvil: a single-center comparative study. Surg Endosc 28:1929–1935
Komatsu S, Ichikawa D, Kashimoto K et al (2013) Risk factors to predict severe postoperative pancreatic fistula following gastrectomy for gastric cancer. World J Gastroenterol 19:8696–8702. doi:10.3748/wjg.v19.i46.8696
Kitano S, Iso Y, Moriyama M et al (1994) Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 4:146–148
Inaba K, Satoh S, Ishida Y et al (2010) Overlap method: novel intracorporeal esophagojejunostomy after laparoscopic total gastrectomy. J Am Coll Surg 211:e25–e29
Nagai E, Ohuchida K, Nakata K et al (2013) Feasibility and safety of intracorporeal esophagojejunostomy after laparoscopic total gastrectomy: inverted T-shaped anastomosis using linear staplers. Surgery 153:732–738
Ebihara Y, Okushiba S, Kawarada Y et al (2013) Outcome of functional end-to-end esophagojejunostomy in totally laparoscopic total gastrectomy. Langenbecks Arch Surg 398:475–479
Tsunoda S, Okabe H, Obama K et al (2014) Short-term outcomes of totally laparoscopic total gastrectomy: experience with the first consecutive 112 Cases. World J Surg. doi:10.1007/s00268-014-2611-2
Nunobe S, Hiki N, Tanimura S et al (2011) Three-step esophagojejunal anastomosis with atraumatic anvil insertion technique after laparoscopic total gastrectomy. J Gastrointest Surg 15:1520–1525
Ichikawa D, Komatsu S, Okamoto K et al (2012) Esophagogastrostomy using a circular stapler in laparoscopy-assisted proximal gastrectomy with an incision in the left abdomen. Langenbecks Arch Surg 397:57–62
Ichikawa D, Komatsu S, Kubota T et al (2013) Effect of hospital volume on long-term outcomes of laparoscopic gastrectomy for clinical stage I gastric cancer. Anticancer Res 33:5165–5170
Acknowledgments
We would like to thank all our colleagues for giving us the opportunity to teach laparoscopic total gastrectomy procedures. Our colleagues are listed below. Kyoto Kizugawa Hospital: Drs. T Obayashi and K Deguchi, and T Daido; Midorigaoka Hospital: Drs. H Tamai and T Imanishi; Kyoto Kujo Hospital: Drs. K Suchi and K Kitagawa; Ayabe City Hospital: Dr. K Inoue; Rakusai Newtown Hospital: Drs. K Imura and N Yamaoka; Kyoto First Red Cross Hospital: Dr. Y Itokawa; Saiseikai Shiga Hospital: Drs. M Masuyama and K Fukuda; Second Okamoto Hospital: Dr. A Iwamoto; Nara City Hospital: Dr. H Nagata; Panasonic Hospital: Dr. N Tani.
Conflict of interest
None to be declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ichikawa, D., Komatsu, S., Kubota, T. et al. Evaluation of the Safety and Feasibility of Laparoscopic Total Gastrectomy in Clinical Stage I Gastric Cancer Patients. World J Surg 39, 1782–1788 (2015). https://doi.org/10.1007/s00268-015-3008-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-015-3008-6