Abstract
Background
Intra-operative nerve monitoring (IONM) of the recurrent laryngeal nerve (RLN) during thyroid and parathyroid surgery is thought to aid in identification and dissection of the RLN. While utilization of IONM is increasing, one area of variability in its application is the assessment of adequate endotracheal tube electrode placement for IONM during the case. The main objective of this study is to assess the overall success of utilizing respiratory variation to confirm proper endotracheal tube placement for RLN monitoring.
Methods
A prospective study of RLN monitoring during thyroid and parathyroid surgery at an academic referral center.
Results
Fifty-five cases were included. Fifty (91 %) achieved optimal respiratory variation during endotracheal tube position. Five (9 %) required repeat laryngoscopy to confirm correct endotracheal tube placement following patient positioning. For the respiratory variation group, average amplitude achieved during initial vagus, maximum vagus, initial RLN, and maximal RLN was 700 (±474) mA, 921 (±616) mA, 887 (±584) mA, and 1330 (±843) mA during evoked stimulation, respectively. For the repeat laryngoscopy group, average amplitude achieved during initial vagus, maximum vagus, initial RLN, and maximal RLN evoked stimulation was 591 (±364) mA, 959 (±306) mA, 771 (±424) mA, and 1462 (±855) mA during evoked stimulation, respectively. There was no statistical difference between the two groups for average initial vagus amplitude (p = 0.62), average maximum vagus amplitude (p = 0.89), average initial RLN amplitude (p = 0.67), or average maximum RLN amplitude (p = 0.74).
Conclusion
The findings of this study support the International Neural Monitoring Study Group recommendation that confirmation of endotracheal tube electrode placement be performed either by confirmation of adequate respiratory variation or by repeat direct laryngoscopy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intra-operative nerve monitoring (IONM) of the recurrent laryngeal nerve (RLN) during thyroid and parathyroid surgery is thought to facilitate early identification of the RLN through neural mapping, aid in RLN dissection, and prognosticate post-operative neural function [1–4]. It is currently estimated that in the US between 44 and 80 % of otolaryngologists and 37–48 % of general surgeons utilize IONM during at least some of their thyroid surgeries, with perhaps a greater utilization seen at academic centers [5–8]. Despite the increased utilization of IONM, it is currently believed that there is a lack of uniformity in the application of IONM that may lead to a varying degree of utility across various surgical venues.
One area of variability in application of IONM is the assessment of adequate endotracheal tube electrode placement during the case. The literature suggests significant neural monitoring inaccuracies derived from nonstandard application of monitoring techniques with a number of recent series documenting significant equipment problems mostly relating to endotracheal tube malposition in 3.8–23 % of IONM patients [9–16]. Dionigi et al. have shown that during initial exposure to neural monitoring 10 % of patients experience setup related problems, including tube rotation (53 %), tube depth/insertion errors (33 %), tube size error (7 %), and displacement of ground electrodes (1 %) [17].
Tube malpositioning is recognized as one of the most common sources of variability in amplitude measurement during monitored surgery [1]. Several methods for assessing adequate endotracheal tube electrode placement have been utilized and include measurement of electrical impedance, assessment of respiratory variation, repeat direct laryngoscopy following patient positioning, and the mechanical “tap test” on the midline thyroid cartilage to assess for neural response. In 2006, with the intention of addressing lack of uniform IONM application, an International Neural Monitoring Study Group was formed in association with the European Society of Endocrine Surgery and the study group recently published a guideline statement for use of IONM [1]. On this issue of optimal tube placement verification technique, this statement suggested a lack of data. However, the IONM group recommended either assessment of respiratory variation or repeat direct laryngoscopy as two approaches that could be utilized. Use of impedance alone or the “tap test” were not recommended. Impedance values represent adequate contact between electrodes and the patient but do not necessarily represent contact specific to the vocal folds. Additionally, the waveforms generated by the “tap test” are poorly understood and could theoretically represent artifactual responses to movements in the electrodes against tissue and do not specifically inform about correct electrode positioning relative to the vocal folds.
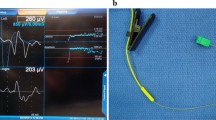
The two techniques to ensure adequate tube electrode placement that have been endorsed by the IONM study group include assessment of respiratory variation or repeat direct laryngoscopy. Respiratory variation is an observed phenomenon that refers to a pattern of coarse small waveforms typically between 30 and 70 μV that can be seen in both monitoring channels if the tube electrodes are in contact with the vocal cords and the patient is in a light plane of anesthesia just prior to spontaneous movement (Fig. 1). It is thought that respiratory variation may reflects the resumption of spontaneous respiratory activity and perhaps even spontaneous phonatory activity when the patient is light from anesthesia, however, the exact neurophysiological basis for the phenomenon remains unclear. The theoretical benefits of this technique include confirmation of intact neural pathway, proof of optimal endotracheal tube placement, as well as less instrumentation when compared with repeat laryngoscopy. If respiratory variation cannot be identified then repeat laryngoscopy is recommended to visually assure adequate endotracheal tube positioning. It is important to note that these tube positional confirmatory tests need to be assessed after the patient is fully positioned with neck extension as this maneuver can alter electrode position relative to the glottis up to 6 cm from the neutral intubating position.
Respiratory variation waveform. a Upper line baseline noise, typically between 10 and 20 μV. Lower line coarsening of the baseline with respiratory variation (occurring in the 30- to 70-μV range when the patient is on the brink of bucking in the early anesthetic period). b Left and right baseline tracings in a patient—the left vocal cord demonstrates normal respiratory variation. The right vocal cord is electrically silent (Patient had known right vocal cord paralysis from past surgery)
The purpose of this project was to prospectively assess the overall success of utilizing respiratory variation to confirm proper endotracheal tube placement in patients undergoing thyroid and parathyroid surgery and to assess correlation between respiratory variation and stimulation of the vagus and RLN.
Materials and methods
With institutional review board approval, 55 consecutive thyroid and parathyroid surgeries performed by a single surgeon (G.W.R.) in a tertiary surgical practice were included. All patients underwent pre-operative laryngoscopy to assess vocal cord function and any patient with an abnormal pre-operative laryngeal examination was excluded. IONM was applied using a standardized IONM set-up using the noninvasive surface electrode endotracheal tube as per guidelines of the International Neural Monitoring Study Group with the Medtronic NIM 2.0 monitor with a surgeon-controlled handheld stimulating probe. The stimulating probe was set to four stimulations per second with a stimulation duration of 100 ms and a supramaximal stimulation between 1 and 2 mA [1]. This system monitors EMG activity in the thyroarytenoid muscle following stimulation of either the vagus or RLN, with EMG depiction visible on the monitor with audio feedback.
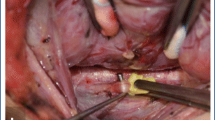
A standardized algorithm for assessing correct endotracheal tube placement was performed in each patient [16]. Anesthesia was induced with a short acting paralyzing agent, and paralytic agents were avoided during the remainder of the case, consistent with international guidelines. The endotracheal tube was inserted under visual control by the anesthesia team to ensure electrode contact with the vocal cords. The patient was then positioned (including placement of shoulder roll, bed positioning, and arm-tucking) while the head and endotracheal tube were held to prevent ETT movement. Following final positioning of the patient, the patient was allowed to briefly lighten from anesthesia to a plane just prior to spontaneous movement in order to confirm the presence of adequate respiratory variation. Adequate respiratory variation is typically defined as coarse small waveforms between 30 and 70 μV that can be seen in both monitoring channels if the tube electrodes are in contact with the vocal cords and the patient is in a light plane of anesthesia just prior to spontaneous movement [1, 18]. For the purpose of this study, coarse waveforms with peak values greater than 30 μV on the side that is being operated were considered confirmatory of adequate respiratory variation. In patients where peak values did not exceed 30 μV, repeat direct laryngoscopy was performed in order to ensure adequate endotracheal tube positioning. Deeper anesthesia was then immediately induced using a nonparalyzing IV agent. Impedance of the electrodes was also checked, with electrode impedance of <5 kΩ and impedance imbalance <1 kΩ. During each case, the vagus nerve and RLN were stimulated with the stimulating probe at 1 mA. IONM parameters that were recorded for the vagus nerve and RLN included both initial and maximum wave amplitudes and compared to the initial respiratory variation values and repeat laryngoscopy data if performed.
Mean values of each parameter for both groups of patients were calculated and compared using a parametric 2-sample Student t test. Significance was set at p value <0.05. Linear regression modeling was performed to identify correlation of maximum respiratory variation values with initial and maximal vagus and RLN amplitudes during stimulation.
Results
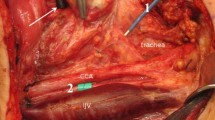
A total of 55 patients met inclusion criteria for the study, including 17 total thyroidectomies, 15 right hemi-thyroidectomies, 19 left hemi-thyroidectomies, and 4 parathyroidectomies (Table 1). Using the algorithm in Fig. 2, 50 patients (91 %) achieved good respiratory variation without requiring repeat direct laryngoscopy (Fig. 3). Five patients (9 %) did not achieve adequate respiratory variation and underwent repeat direct laryngoscopy to confirm correct endotracheal tube placement following patient positioning. There was no difference in age (p = 0.84), gender (p = 0.72), or type of surgery (p = 0.71–0.87) between patients achieving adequate respiratory variation and those requiring repeat laryngoscopy.
Both the respiratory variation group and the repeat laryngoscopy group achieved adequate initial and maximum vagus and RLN responses to evoked stimulation. For the respiratory variation group, average amplitude achieved during initial vagus, maximum vagus, initial RLN, and maximal RLN was 700 (±474) mA, 921 (±616) mA, 887 (±584) mA, and 1330(±843) mA during evoked stimulation, respectively. For the repeat laryngoscopy group, average amplitude achieved during initial vagus, maximum vagus, initial RLN, and maximal RLN-evoked stimulation was 591 (±364) mA, 959(±306) mA, 771 (±424) mA, and 1462 (±855) mA during evoked stimulation, respectively. (Table 2) There was no statistical difference between the two groups for average initial vagus amplitude (p = 0.62), average maximum vagus amplitude (p = 0.89), average initial RLN amplitude (p = 0.67), or average maximum RLN amplitude (p = 0.74).
In the respiratory variation group, there did not appear to be good linear correlation between maximum amplitude of respiratory variation achieved and initial or maximal vagus and RLN amplitudes on evoked stimulation. Figure 4 demonstrates linear regression modeling of maximum amplitude achieved during respiratory variation and intra-operative evoked responses of the vagus nerve and RLN. The coefficient of determination (r 2) for assessing maximum respiratory variation amplitude and initial vagus, maximum vagus, initial RLN, and maximum RLN amplitude using simple linear regression was 0.118, 0.094, 0.053, and 0.047, respectively.
Correlation of respiratory variation with IONM parameter graph a, b, c, and d represent maximum respiratory variation amplitude achieved prior to the start of each case (Y-axis) versus initial vagus nerve amplitude (a), maximum vagus nerve amplitude (b), initial recurrent laryngeal nerve amplitude (c), and maximum recurrent laryngeal nerve amplitude (d)
Discussion
At our institution, the algorithm for set-up of IONM remains assessment of respiratory variation to determine endotracheal tube electrode position followed by direct laryngoscopy if respiratory variation is not achieved (Fig. 2). In this study, adequate respiratory variation was achieved in 91 % of patients and predicted adequate initial vagus and RLN responses in all patients. When adequate respiratory variation was not achieved, as was the case with 9 % of patients of this study, repeat direct laryngoscopy to visualize ETT position predicted adequate initial vagus and RLN responses in all cases. These findings strongly support IONM Study Group recommendations of using either technique for assessment of ETT placement.
Our vagal and RLN-evoked stimulation were similar and adequate in both the respiratory variation and repeat laryngoscopy groups suggesting that these are two alternate pathways both of which are successfully associated with optimal tube placement and robust IONM waveform parameters during surgery. Despite lack of statistical significance between methods for assessing correct placement of endotracheal tube pre-operatively, the authors of this study support routine use of respiratory variation over repeat direct laryngoscopy when possible. The theoretical benefits of achieving adequate respiratory variation include confirmation of intact neural pathway as well as less instrumentation when compared with repeat laryngoscopy.
In our experience, when respiratory variation is not achieved, the endotracheal tube is found to be malposition on repeat direct laryngoscopy roughly 50 % of the time and in good position roughly 50 % of the time (unpublished data). As a result, the authors speculated that absence of respiratory variation perhaps has at least two sources: (1) a malpositioned endotracheal tube and (2) a mechanism whereby emergence from anesthesia does not evoke respiratory variation for some patients. It is important to recognize that this occurs in less than 10 % of the studied cases.
Although respiratory variation predicted good intra-operative evoked vagus and RLN response in this study, the exact relationship of respiratory variation and intra-operative evoked response is less clear. In the respiratory variation group, 100 % of cases meeting IONM Guidelines for adequate respiratory variation demonstrated sufficient intra-operative evoked response. Despite this predictive success, there did not appear to be good linear correlation between maximum amplitude of respiratory variation identified during endotracheal tube positioning and initial or maximal vagus and RLN amplitudes on evoked stimulation seen in Fig. 4. Given these findings, we believe it is reasonable to assume adequate electrode placement if maximum respiratory variation amplitudes are greater than 30 mA, however, the exact value achieved does not appear to predict the evoked responses that one might then see intra-operatively.
As previously mentioned, equipment problems relating to endotracheal tube malposition have been seen in 3.8–23 % of monitored patients [9–16]. Our study identified 9 % of patients requiring repeat direct laryngoscopy to assess adequate electrode placement, with sufficient intra-operate evoked responses seen in each (100 %) of these cases following visual endotracheal tube electrode position confirmation. These findings suggest that repeat direct laryngoscopy following final positioning of the patient remains a reliable strategy for confirmation of electrode position.
IONM of the RLN has been used to facilitate early neural identification of the RLN through neural mapping, aid in dissection once the RLN is identified, prognosticate post-operative neural function and has been applied to the RLN as well as the external branch of the SLN [19]. We believe that the application of standardized monitoring techniques will only make IONM a more effective tool for thyroid and parathyroid surgeons. The exact learning curve to master IONM is likely different for each surgeon, however, several studies suggest nerve monitoring may be mastered between 50 and 100 cases [17, 20, 21], with one study showing reduction in equipment related problems to 0 % with routine application of IONM [4]. One central element of successful IONM technique remains the adequate set-up and confirmation of endotracheal tube electrode placement. For this, close partnership with anesthesiologist is essential [17, 22].
Conclusion
IONM of RLN has increased during thyroid and parathyroid surgery both by otolaryngologists and general surgeons to facilitate early neural identification of the RLN through neural mapping, aid in dissection once the RLN is identified, and prognosticate post-operative neural function. Application of monitoring standards is crucial to the success of translating nerve monitoring into enhanced patient safety. The findings of this study support the International Neural Monitoring Study Group recommendation that confirmation of endotracheal tube electrode placement be performed either by confirmation of adequate respiratory variation or by repeat direct laryngoscopy.
Limitation
-
1.
Small study.
-
2.
Highly skilled anesthesia staff required, so we understand it may not be possible to employ respiratory variation in all settings.
References
Randolph GW, Dralle H, Abdullah H et al (2011) Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope 121(Suppl 1):S1–S16
Dralle H, Sekulla C, Lorenz K et al (2008) Intraoperative monitoring of the recurrent laryngeal nerve in thyroid surgery. World J Surg 32:1358–1366. doi:10.1007/s00268-008-9483-2
Snyder SK, Lairmore TC, Hendricks JC et al (2008) Elucidating mechanisms of recurrent laryngeal nerve injury during thyroidectomy and parathyroidectomy. J Am Coll Surg 206:123–130
Chiang FY, Lee KW, Chen HC et al (2010) Standardization of intraoperative neuromonitoring of recurrent laryngeal nerve in thyroid operation. World J Surg 34:223–229. doi:10.1007/s00268-009-0316-8
Horne SK, Gal TJ, Brennan JA (2007) Prevalence and patterns of intraoperative nerve monitoring for thyroidectomy. Otolaryngol Head Neck Surg 136:952–956
Sturgeon C, Sturgeon T, Angelos P (2009) Neuromonitoring in thyroid surgery: attitudes, usage patterns, and predictors of use among endocrine surgeons. World J Surg 33:417–425. doi:10.1007/s00268-008-9724-4
Singer MC, Rosenfeld RM, Sundaram K (2012) Laryngeal nerve monitoring: current utilization among head and neck surgeons. Otolaryngol Head Neck Surg 146:895–899
Ho Y, Carr MM, Goldenberg D (2013) Trends in intraoperative neural monitoring for thyroid and parathyroid surgery amongst otolaryngologists and general surgeons. Eur Arch Otorhinolaryngol 270:2525–2530
Dralle H, Sekulla C, Haerting J et al (2004) Risk factors of paralysis and functional outcome after recurrent laryngeal nerve monitoring in thyroid surgery. Surgery 136:1310–1322
Echeverri A, Flexon PB (1998) Electrophysiologic nerve stimulation for identifying the recurrent laryngeal nerve in thyroid surgery: review of 70 consecutive thyroid surgeries. Am Surg 64:328–333
Eltzschig HK, Posner M, Moore FD Jr (2002) The use of readily available equipment in a simple method for intraoperative monitoring of recurrent laryngeal nerve function during thyroid surgery: initial experience with more than 300 cases. Arch Surg 137:452–456 discussion 456–457
Snyder SK, Hendricks JC (2005) Intraoperative neurophysiology testing of the recurrent laryngeal nerve: plaudits and pitfalls. Surgery 138:1183–1191 discussion 1191–1182
Shindo M, Chheda NN (2007) Incidence of vocal cord paralysis with and without recurrent laryngeal nerve monitoring during thyroidectomy. Arch Otolaryngol Head Neck Surg 133(481–48):5
Hermann M, Hellebart C, Freissmuth M (2004) Neuromonitoring in thyroid surgery: prospective evaluation of intraoperative electrophysiological responses for the prediction of recurrent laryngeal nerve injury. Ann Surg 240:9–17
Beldi G, Kinsbergen T, Schlumpf R (2004) Evaluation of intraoperative recurrent nerve monitoring in thyroid surgery. World J Surg 28:589–591. doi:10.1007/s00268-004-7226-6
Lu IC, Chu KS, Tsai CJ et al (2008) Optimal depth of NIM EMG endotracheal tube for intraoperative neuromonitoring of the recurrent laryngeal nerve during thyroidectomy. World J Surg 32:1935–1939. doi:10.1007/s00268-008-9549-1
Dionigi G, Bacuzzi A, Boni L et al (2008) What is the learning curve for intraoperative neuromonitoring in thyroid surgery? Int J Surg 6(Suppl 1):S7–S12
Macias AES, O’Neil E, Kamani D, Malikin I, Konowitz P, Randolph GW (2014) Intraoperative electrophysiologic monitoring of the recurrent laryngeal nerve during thyroid and parathyroid surgery : The Massachusetts Eye and Ear Infirmary Monitoring Protocol with Collaborative Experience in over 3000 cases. Br J Anesth (submitted)
Barczyński M, Randolph GW, Cernea CR et al (2013) External branch of the superior laryngeal nerve monitoring during thyroid and parathyroid surgery: international neural monitoring study group standards guideline statement. Laryngoscope 123:S1-S14
Duclos A, Lifante JC, Ducarroz S et al (2011) Influence of intraoperative neuromonitoring on surgeons’ technique during thyroidectomy. World J Surg 35:773–778. doi:10.1007/s00268-011-0963-4
Dralle H et al (2004) What benefits does neural monitoring bring to thyroid surgery? Artz Krankenh 12:369–376
Deiner S (2010) Highlights of anesthetic considerations for intraoperative neuromonitoring. Semin Cardiothorac Vasc Anesth 14:51–53
Acknowledgments
This work was funded by the John and Claire Bertucci Thyroid Research fund.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chambers, K.J., Pearse, A., Coveney, J. et al. Respiratory Variation Predicts Optimal Endotracheal Tube Placement for Intra-operative Nerve Monitoring in Thyroid and Parathyroid Surgery. World J Surg 39, 393–399 (2015). https://doi.org/10.1007/s00268-014-2820-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-014-2820-8