Abstract
Background
Malpositioning of the endotracheal surface electrodes can result in dysfunction of intraoperative neuromonitoring (IONM) and increase the risk of recurrent laryngeal nerve injury. The purpose of this study was to investigate the optimal depth of the nerve integrity monitor (NIM) EMG endotracheal tube.
Methods
We enrolled 105 adult patients undergoing elective thyroidectomy. Each Medtronic Xomed NIM EMG endotracheal tube was placed with the middle of the exposed electrodes well in contact with the true vocal cords under direct laryngoscopy. Function of IONM was documented and the insertion depth was measured and analyzed.
Results
Ninety-nine (94.3%) patients had successful IONM with the initial endotracheal tube position. Six (5.7%) patients needed further tube depth adjustment under fiberoptic bronchoscopy. All patients were finally had successful IONM. The optimal mean depth was 20.6 ± 0.97 cm in men and 19.6 ± 1.0 cm in women (p < 0.01). There was the trend that taller subjects had a deeper tube depth (p < 0.05).
Conclusion
We concluded that the mean depth of the NIM EMG tube would be a useful reference value for detecting the malposition of electrodes and adjusting the depth of tube during the operation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intraoperative neuromonitoring (IONM) was proposed to verify the functional integrity of recurrent laryngeal nerve (RLN) [1–14] during thyroid surgery. Several neuromonitoring instrument setups have been developed in the past three decades. These can be subdivided into four separate techniques: needle electrodes inserted into vocalis muscles by direct laryngoscopy or through the cricothyroid ligament, and surface electrodes placed against the postcricoid area or with endotracheal tubes that have attached electrodes. In recent years, endotracheal tube surface electrodes have gained more popularity because of the essential advantages of their ease of setup and use, their noninvasive nature, and their capacity to derive larger areas of the target muscle. However, the surface electrodes may become malpositioned after the neck is fully extended for the operation, although the vocal cords may actually be seen in contact with the tube electrodes at initial intubation. In some patients the relative position between the exposed electrodes and vocal cords may not be clearly identified because of the poor view under direct laryngoscopy.
Malposition of the tube can result in equipment failure or unsuccessful monitoring which could in turn potentially give misleading information that might increase the risk of RLN injury. The purpose of this study was to investigate the optimal depth of Medtronic Xomed Nerve Integrity Monitor (NIM) EMG Endotracheal Tube (Jacksonville, FL) for IONM; this would help to detect and resolve equipment failure caused by malpositioning of the tube.
Materials and methods
The study was approved by the ethics committee of the medical faculty of the Kaohsiung Medical University, Taiwan, and written informed consent was obtained from each patient. Patients were informed of the intent to use this monitoring system potentially to aid in the localization and identification of the RLNs and assessment of their function during operation. There was no financial or professional association between the authors and the commercial company whose nerve monitoring product was studied.
From April 2006 to June 2007, 105 adult patients (30 men and 75 women; ages range = 23-78 years; mean age = 50.6 years) undergoing operations for various thyroid diseases and all being treated by the same surgeon were enrolled in this study. There were 40 total lobectomies and 65 total thyroidectomies. Four nerves were excluded from this study (three nerves featured preoperative cord palsy and one nerve was sacrificed intentionally due to cancer invasion), so in total 166 nerves at risk were enrolled in this study.
The patient’s head and neck were kept at a neutral position during tracheal intubation. Following induction of anesthesia with fentanyl 2 μg/kg and thiopenthal 5 mg/kg, quantitative monitoring of the neuromuscular blockade with the relaxometry (TOF-GUARD system, Organon Teknika, Belgium) was established at this time. After stable train-of-four data from relaxometry (100% without using a muscle relaxant) for 60 s, all patients were given a standard dose of muscle relaxant. When maximal neuromuscular blockade was achieved, a Medtronic Xomed NIM EMG endotracheal tube was inserted by the same anesthesiologist. The tube was placed with the middle of the blue-marked region (the exposed electrodes) well in contact with the true vocal cords under direct laryngoscopy. Then the tube was fixed at the right mouth angle. The optimal depth was defined at subsequent successful IONM. The insertion depth was measured from the tip of the tube to the right corner of the mouth. Rotation of NIM EMG endotracheal tube could be detected by EMG impedance and corrected manually. Anesthetic care was provided by the same anesthesia nurse. No additional muscle relaxant was given after the first administration. Cuff pressure was kept at 20–22 cmH2O.
After changing the position of the head and neck from neutral to full extension, we checked monitor function and endotracheal tube electrode position by assessing the following: (1) impedance values of less than 5 kΩ and impedance imbalance of less than 1.0 kΩ, and (2) the presence of normal baseline. The position of the head and neck was kept fully extended until the end of the operation.
The vagus nerve was routinely stimulated before identification of the RLN, and a successful IONM was defined as the signals from the vagus nerve and the RLN obtained during the operation when the nerve stimulator made direct contact with the nerve at a level of 0.80 mA. All IONM signals were controlled by EMG documentation at the same time to exclude artifact signals. The intensity of the electric current was set at 0.5 mA as a starting point and event threshold at 100 μV; if no signal was elicited, the intensity was increased to 0.8 mA. Equipment failure would be considered if signals from the vagus nerve could not be obtained at a level of 1 mA. After the RLN was completely dissected from Berry’s ligament, the EMG signals from the RLN and the vagus nerve were compared with the original data obtained before RLN dissection.
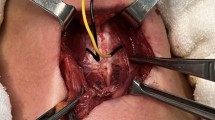
All patients received a preoperative and postoperative video recording of vocal cord movement with flexible laryngofiberoscopy, and all exposed RLNs were photographically documented with a high-resolution camera to show visual nerve integrity during the operation. When vocal cord dysfunction was identified, follow-up was arranged for every 2 weeks initially and every 4 weeks thereafter until recovery was achieved. Dysfunction was considered permanent if it persisted 6 months after surgery.
Analysis of the mean depth of the NIM EMG endotracheal tube was performed using the t test for male and female subjects. Multiple regression analysis was carried out to analyze the influence of age, body weight, body height, and body mass index (BMI) on the optimal depth. Subjects were divided into four groups based on body height (<151 cm, 151–160 cm, 161–170 cm, and >171 cm). Analysis of variance was performed to analyze the effect of body height on tube insertion depth. SPSS package software (SPSS Inc., Chicago, IL) was used for the analyses. For multiple linear regression analysis, the significant level for a variable was set at α = 0.05. P < 0.05 was considered statistically significant. Results are presented as mean ± SD (standard deviation).
Results
Demographic data and mean tube depth of 105 adult patients are given in Table 1. The mean depth was 20.6 ± 0.97 cm in men, which was significantly deeper than the 19.6 ± 1.0–cm depth in women (p < 0.01). The relationship between body height and tube depth is given in Table 2. There was a trend that subjects with greater body height had deeper tube depth (p < 0.05). Other demographic variables such as age, body weight, and BMI were not significantly correlated to the optimal depth (p > 0.05).
Ninety-nine (94.3%) patients had successful IONM after initial tracheal intubation, but six (5.7%) patients needed further tube depth adjustment, in three the tube was inserted too far, and in the other three it was too shallow under fiberoptic bronchoscopic examinations. All patients were finally successful under IONM. Table 3 gives the depths of the NIM EMG endotracheal tube before and after adjustment in those with dysfunction of neuromonitoring.
Nine nerves lost EMG signals after complete RLN dissection and the signals did not recover before the wound was closed; all developed temporary cord palsy.
Discussion
RLN palsy is the most common and serious complication after thyroid surgery. Surgeons attempt to reduce the palsy rates by identifying the RLN [15–17] or by peforming IONM during the operation. Several reports [5, 8, 18] have advocated that IONM was a useful and reliable tool for identification and preservation of the RLN, although the value of reducing the RLN palsy rates remained controversial [1, 3, 5, 7]. We selected the EMG monitoring approach with a commercially prepared endotracheal tube—Medtronic Xomed Nerve Integrity Monitor (NIM) EMG Endotracheal Tube—and it was set up routinely for every patient undergoing thyroid surgery starting in April 2006. We used this monitoring system to locate and identify the RLNs, assess their function during the operation, and elucidate the possible mechanism of RLN injury.
The endotracheal tube laryngeal surface electrodes have the following important advantages: ease of setup and use, noninvasive nature, and ability to sample greater regions of evoked muscle action potentials; however, any improper connection of wires or tube rotation or displacement of the electrodes could result in equipment failure. Improper connection of the wires and tube rotation could be detected and corrected easily by checking the monitor with the presence of a normal baseline and with the impedance imbalance less than 1.0 kΩ. However, the position of the electrodes could be displaced and not be detected when the patient’s head and neck position is changed from neutral for tracheal intubation to full extension for thyroid surgery. Yep et al. [19] found that for any given position change in a patient, the direction and magnitude of endotracheal tube displacement would be unpredictable. A range of endotracheal tube displacement from 21 mm inward and 33 mm outward has been reported after neck extension. The wide range of displacement found on flexion and extension may result from changes in soft tissue geometry on the endotracheal tube and the mobile lung root. Changes in tracheal tube position during head and neck position change have been reported for both adults and children. Kim’s study [20] revealed that after pneumoperitoneum in a patient in the Trendelenburg position, the tracheal length was decreased by 0.42 ± 0.19 cm and the endotracheal tube tip was displaced 0.85 ± 0.25 cm inward. This effect might increase the risk of accidental endobronchial intubation during gynecologic laparoscopic surgery. Jin-Hee’s study [21] on children between 2 and 8 years revealed that full neck extension resulted in tracheal elongation of 0.95 ± 0.43 cm and displacement of the endotracheal tube tip outward to the vocal cords by 1.08 ± 0.47 cm. The reported range was relatively wide compared with 3 cm of exposed electrodes, and this change could possibly lead to malposition of electrodes and therefore result in equipment failure.
Dysfunction of IONM setup has been reported in several studies. In the series of Beldi et al. [22], the monitoring failed in 39 (23%) procedures because of technical problems in 296 operations. In the series of Chan and Lo [3], there were five patients with equipment failure of unknown causes and these were excluded from their study. In Snyder and Hendricks’s series [5], there were nerve monitoring setup problems in 3.8% of cases (7/185), four of which were of unknown causes. They suggested that correct positioning of the endotracheal tube electrodes against each vocal cord be confirmed on the nerve integrity monitor (NIM) unit by demonstrating an impedance imbalance of less than 1.0 kΩ for each wire.
In our study, six (5.7%) patients developed a problem with the IONM even though all showed a normal baseline and an impedance imbalance of less than 1.0 kΩ. In three, insertion was too deep, and for the other three, it was too shallow under fiberoptic examination and required further insertion depth adjustments. All six patients had successful IONM after tube depth adjustment. We found that an impedance imbalance of less than 1.0 kΩ and the presence of a normal baseline meant only that all the wires were well connected and the endotracheal tube was not rotated; it did not necessarily mean the IONM was working. Among the six patients who had a IONM problem, in five it was detected when the EMG signal could not be obtained by stimulating the vagus nerve. In the sixth patient (tube depth = 21 cm) a signal was initially obtained from the vagus nerve, but the EMG signal was close to the trachea when localization of the RLN was performed. Although the electrodes were well in contact with the vocal cords, the tube was too far into the trachea so that stimulation close to the trachea produced an EMG response. We withdrew the tube 1 cm upward and the problem was resolved. Table 3 gives the depth of tube before and after adjustment in those with dysfunction of the IONM; we found that the depth after adjustment was close to our mean depth.
The relationship between body height and tube depth is given in Table 2. There was a trend that the subjects in the greater body height group had deeper insertion depths. Cherng et al. [23] reported a similar result by which the optimal insertion depth for orotracheally intubated adult patients was correlated with body height.
In this study we found that the mean depth of the NIM EMG endotracheal tube could provide a very useful reference value to the IONM user. First, malposition of electrodes could be suspected before preparation for surgery if the depth of he NIM EMG tube was far from our mean depth. It would be troublesome and require time to check and adjust the position of the EMG endotracheal tube when malposition was considered during the operation. Second, it helps to judge whether the tube is inserted too deeply or not deep enough during the operation. It is not necessary to check the tube position routinely with a fiberoptic bronchoscope. In our experience, the only thing we have to do is adjust the tube to be close to our mean depth according to gender and body height. Third, it provides a reference value for insertion depth when a poor laryngoscopic view is encountered. In this study, there were poor laryngoscopic views during insertion of the EMG tube in three male and four female patients (total 6.7%). Six were classified as Modified Cormark-Lehane Score [24, 25] grade 2B and one was grade 3. All seven patients were successfully intubated at the first attempt according to our mean depth. The insertion depth of the NIM EMG endotracheal tube for those seven patients was rechecked by fiberoptic bronchoscopy and none showed dysfunction of the IONM.
We routinely stimulated the vagus nerve before identification of the RLN by directly touching the nerve with the nerve stimulator (Prass monopolar probe). The intensity of the electric current was set at 0.5 mA as a starting point and event threshold at 100 μV; if no signal was elicited, the intensity was increased to 0.8 mA. Equipment failure would be considered if signals from the vagus nerve could not be obtained at a level of 1 mA. There are several advantages to vagus nerve stimulation: (1) it could confirm that the monitoring system was working; the surgeon would be much more confident when testing the RLN; (2) it could provide original data such as the level of current and the intensity of evoked potential before dissecting the RLN. The level we used to elicit an EMG response from the vagus nerve can definitely elicit a response from RLN; (3) it could provide data that would be very useful to compare the signals before and after dissection of RLN; and (4) it could offer another way to verify the status of the RLN when the RLN was very difficult to identify.
In our daily practice, the EMG endotracheal tube was fixed at the right mouth angle of the patient after oral tracheal intubation; this is much easier than maintaining the tube in midline placement. NIM EMG endotracheal tubes might run in a clockwise rotation when fixed to the right mouth angle. Rotation of the tube could be prevented by rotating it counterclockwise about 30°. Rotation can also be detected easily by checking the impedance imbalance of the NIM EMG monitor.
When signals were lost or weakened compared with the original signals after complete nerve dissection, this meant that the RLN had been injured during nerve dissection and we routinely located the disrupted point of conduction. In this study we found that IONM helped elucidate the mechanism of nerve injury during RLN preparation. During the learning curve of IONM, it might not be as beneficial as visual identification only, but it would provide instructive information for future operations.
In conclusion, IONM requires good cooperation with the anesthesiologist. With respect to the NIM EMG endotracheal tube for IONM, malposition of the electrodes was the main cause of equipment failure. The displacement of the electrodes could remain undetected by the NIM monitor when the patient’s head and neck position is changed from the neutral position for tracheal intubation to full extension for thyroid surgery.
Surgeons who use this technology should be familiar with the setup of the IONM and understand its potential pitfalls with respect to its improper setup, equipment failure, and the possible confusing results to avoid inflicting harm on the RLN. In this study, the mean depth of the NIM EMG endotracheal tube would be a useful reference value; it could not only help in detection of the malpositioning of electrodes before operation, but it could also be used to adjust the depth of the tube during the operation.
References
Shindo M, Chheda NN (2007) Incidence of vocal cord paralysis with and without recurrent laryngeal nerve monitoring during thyroidectomy. Arch Otolaryngol Head Neck Surg 133:481–485
Tomoda C, Hirokawa Y, Uruno T et al (2006) Sensitivity and specificity of intraoperative recurrent laryngeal nerve stimulation test for predicting vocal cord palsy after thyroid surgery. World J Surg 30:1230–1233
Chan WF, Lo CY (2006) Pitfalls of intraoperative neuromonitoring for predicting postoperative recurrent laryngeal nerve function during thyroidectomy. World J Surg 30:806–812
Witt RL (2005) Recurrent laryngeal nerve electrophysiologic monitoring in thyroid surgery: the standard of care? J Voice 19:497–500
Snyder SK, Hendricks JC (2005) Intraoperative neurophysiology testing of the recurrent laryngeal nerve: plaudits and pitfalls. Surgery 138:1183–1191
Pearlman RC, Isley MR, Ruben GD et al (2005) Intraoperative monitoring of the recurrent laryngeal nerve using acoustic, free-run, and evoked electromyography. J Clin Neurophysiol 22:148–152
Yarbrough DE, Thompson GB, Kasperbauer JL et al (2004) Intraoperative electromyographic monitoring of the recurrent laryngeal nerve in reoperative thyroid and parathyroid surgery. Surgery 136:1107–1115
Randolph G.W, Kobler JB, Wilkins J (2004) Recurrent laryngeal nerve identification and assessment during thyroid surgery: laryngeal palpation. World J Surg 28:755–760
Dralle H, Sekulla C, Haerting J et al (2004) Risk factors of paralysis and functional outcome after recurrent laryngeal nerve monitoring in thyroid surgery. Surgery 136:1310–1322
Marcus B, Edwards B, Yoo S et al (2003) Recurrent laryngeal nerve monitoring in thyroid and parathyroid surgery: the University of Michigan experience. Laryngoscope 113:356–361
Tschopp KP, Gottardo C (2002) Comparison of various methods of electromyographic monitoring of the recurrent laryngeal nerve in thyroid surgery. Ann Otol Rhinol Laryngol 111:811–816
Otto RA, Cochran CS (2002) Sensitivity and specificity of intraoperative recurrent laryngeal nerve stimulation in predicting postoperative nerve paralysis. Ann Otol Rhinol Laryngol 111:1005–1007
Djohan RS, Rodriguez HE, Connolly MM et al (2000) Intraoperative monitoring of recurrent laryngeal nerve function. Am Surg 66:595–597
Eisele DW (1996) Intraoperative electrophysiologic monitoring of the recurrent laryngeal nerve. Laryngoscope 106:443–449
Karlan MS, Catz B, Dunkelman D et al (1984) A safe technique for thyroidectomy with complete nerve dissection and parathyroid preservation. Head Neck Surg 6:1014–1019
Jatzko GR, Lisborg BH, Muller MG et al (1994) Recurrent nerve palsy after thyroid operations—principal nerve identification and a literature review. Surgery 115:139–144
Chiang FY, Wang LF, Huang YF et al (2005) Recurrent laryngeal nerve palsy after thyroidectomy with routine identification of the recurrent laryngeal nerve. Surgery 137:342–347
Thomusch O, Sekulla C, Machens A et al (2004) Validity of intra-operative neuromonitoring signals in thyroid surgery. Langenbecks Arch Surg 389:499–503
Yap SJ, Morris RW, Pybus DA (1994) Alterations in endotracheal tube position during general anaesthesia. Anaesth Intensive Care 22:586–588
Kim JH, Hong DM, Oh AY et al (2007) Tracheal shortening during laparoscopic gynecologic surgery. Acta Anaesthesiol Scand 51:235–238
Jin-Hee K, Ro YJ, Seong-Won M et al (2005) Elongation of the trachea during neck extension in children: implications of the safety of endotracheal tubes. Anesth Analg 101:974–977
Beldi G, Kinsbergen T, Schlumpf R (2004) Evaluation of intraoperative recurrent nerve monitoring in thyroid surgery. World J Surg 28:589–591
Cherng CH, Wong CS, Hsu CH et al (2002) Airway length in adults: estimation of the optimal endotracheal tube length for orotracheal intubation. J Clin Anesth 14:271–274
Cormack RS, Lehane J (1984) Difficult tracheal intubation in obstetrics. Anaesthesia 39:1105–1111
Koh LK, Kong CE, Ip-Yam PC (2002) The modified Cormack-Lehane score for the grading of direct laryngoscopy: evaluation in the Asian population. Anaesth Intensive Care 30:48–51
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, IC., Chu, KS., Tsai, CJ. et al. Optimal Depth of NIM EMG Endotracheal Tube for Intraoperative Neuromonitoring of the Recurrent Laryngeal Nerve During Thyroidectomy. World J Surg 32, 1935–1939 (2008). https://doi.org/10.1007/s00268-008-9549-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-008-9549-1