Abstract
Introduction
The diagnosis and treatment of hilar tumors requires a multidisciplinary approach based on the synergy of radiologists, surgeons, oncologists, and gastroenterologists. Klatskin tumor is a relatively rare disease with a poor prognosis. Currently, the only possible treatment is represented by the removal of the tumor associated with radical surgery, even though its results are still jeopardized by significant morbidity and mortality. A proper preoperative optimization of the patient, including staging laparoscopy, biliary drainage, and portal vein embolization, may improve short-term outcome. The purpose of this study was to evaluate the short- and long-term impact of preoperative optimization in patients affected by hilar cholangiocarcinoma.
Methods
From January 2004 to May 2012, 94 patients with preoperative diagnosis of Klastkin tumors were candidates for surgery at the Hepatobiliary Surgery Unit of the Hospital San Raffaele in Milan. The data of all patients were prospectively collected and retrospectively reviewed. The outcome was evaluated in terms of perioperative morbidity and mortality and overall and disease-free survival. Short-term outcome of patients undergoing preoperative optimization was compared with outcome of patients who did not undergo it in terms of intraoperative data, morbidity and mortality.
Results
Of 94 patients undergoing surgery, 80 underwent hepatic and biliary confluence resection. Fourteen patients were considered unresectable due to the presence of peritoneal carcinomatosis or advanced disease seen during staging laparoscopy or at laparotomy and therefore were excluded from the analysis. Seventy-five (93.7 %) patients underwent major liver resections: in 14 of these, surgery was performed at a distance of 30–40 days from PVE. In 55 patients, biliary drainage was preoperatively placed for palliation of obstructive jaundice. The postoperative morbidity rate was 51.2 % and mortality 6.2 %. The most frequent cause of death was postoperative liver failure. Five-year survival rate was 29 %. Patients undergoing preoperative optimization experienced a significant reduction of postoperative morbidity, especially in terms of infectious related events.
Conclusions
Klatskin tumor remains a disease associated with poor prognosis, but a correct preoperative diagnostic and therapeutic management provides tools to perform this type of surgery with acceptable morbidity and mortality, thus improving long-term results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hilar cholangiocarcinoma is a relatively uncommon neoplasm originating from the biliary confluence or from the left/right hepatic duct [1]; tumor biology, along with its position close to liver parenchyma and to vascular hilar structures, makes its management challenging. Surgical resection is the only potentially curative treatment for hilar cholangiocarcinoma: obtaining negative margins, often acquired only with associated major liver resection (to manage both direct parenchymal invasion and longitudinal extension of the disease along the ducts), is one of the strongest prognostic factors to provide better long-term survival [2].
In this setting, morbidity and mortality rates are significant, resulting mainly from liver failure and infections, because biliary and associated liver surgery are frequently complex and imply extensive demolitions of healthy liver to obtain a radical resection [3–6]. A better patient selection and improved preoperative optimization of candidates through multidisciplinary management, consisting of staging laparoscopy to assess resectability, biliary drainage to treat intrahepatic biliary cholestasis, and portal vein embolization to increase future liver remnant volume, may contribute to improve short-term outcome [7–9]. The purpose of this study was to analyze the impact of preoperative optimization in patient candidates to major liver resections for hilar cholangiocarcinoma.
Patients and methods
Between January 2004 and May 2012, 1,340 liver resections have been performed at the Hepatobiliary Surgery Unit of San Raffaele Hospital in Milan. Data from these patients have been collected in a prospective database and are now retrospectively reviewed.
In this period, 94 patients were referred to the same Centre for hilar cholangiocarcinoma and were candidates to surgery, representing the study population. Patients affected by unresectable hilar cholangiocarcinoma were excluded from this series (resectability criteria are mentioned below), as well as those affected by gallbladder cancer and peripheral cholangiocellular tumors.
Preoperative evaluation
Before surgery, all patients were evaluated by thoracoabdominal imaging [computed tomography (CT) and magnetic resonance cholangiopancreatography (MRCP)], blood tests, including serum tumor markers levels [carcinoembryonic antigen (CEA) and Ca 19.9]. The side of hepatectomy was evaluated according to the predominant infiltration of the disease along the biliary tree and toward vascular hilar structures. Selected patients also underwent positron emission tomography (PET) to evaluate presence of extrahepatic disease. Preoperative chemotherapy was not routinely administrated. Diagnosis assessed by cytology was not a prerequisite for surgery. Treatment strategies were systematically evaluated at weekly multidisciplinary meetings, including liver surgeons, radiologists, and medical oncologists, to define the final indication for the surgical procedure and both the type and the resection technique.
Tumor classification and histopathological examination
The Bismuth–Corlette classification [7] was used to define preoperatively tumor extension along the intrahepatic bile ducts, taking into account findings from imaging. Intraoperatively, tumor extension and margins were examined by histopathological evaluation of frozen sections. The histological staging of the disease was determined according to the TNM classification following the criteria of the American Joint Committee on Cancer (AJCC) [8]. Resections were considered curative (R0) when margins had no evidence of microscopic disease.
Criteria for resectability
Resectability was defined according to MSKCC staging system [9]. Patients with locally advanced disease involving secondary biliary branches of both sides or the main portal vein proximally to its bifurcation were excluded from surgery. Atrophy of one hepatic lobe or unilateral tumor extension to secondary order biliary branches with contralateral portal vein or arterial involvement also was considered a contraindication to surgery. Nodes involvement was not considered an absolute surgical contraindication, except for metastases located beyond the hepatoduodenal ligament [9].
Preoperative optimization
Liver optimization consisted of preoperative percutaneous transhepatic biliary drainage (PTBD) and preoperative portal vein embolization (PVE) for patients whose future liver remnant (FLR) was judged inadequate. Adopted algorithm is summarized in Fig. 1.
Staging laparoscopy was performed routinely in candidates to preoperative PTBD and PVE to exclude the presence of peritoneal carcinomatosis, extrahepatic metastases, and locally advanced hilar disease and to confirm patient suitability to surgery. In other cases, staging laparoscopy was performed at the beginning of the surgical intervention to reduce the number of useless laparotomies.
Indication for PTBD placement was obstructive jaundice or imaging evidence of bile ducts dilatation. Endoscopic drainage was avoided whenever possible. External PTBD was preferred to external–internal drainage and bile replacement was never performed. Drainage of the FLR was considered as a first choice, even though in selected cases the hemiliver that had to be removed was drained as well to achieve jaundice relief and bile duct distension. Surgery was postponed until total bilirubin level was <5 mg/dL.
Computed tomography (CT) study was used to determine liver volumes by 3D reconstruction of images. Total liver volume (TLV), tumor volume, and resected volume were calculated before embolization using dedicated software, multiplying the area of each liver session by the slice thickness. The FLR was estimated subtracting tumor volume as follows: (resected volume − tumor volume)/(TLV − tumor volume). PVE was indicated when the predicted FLR was <30 % in patients with normal parenchyma or <40 % in patients with abnormal parenchyma due to obstructive cholestasis or chronic liver diseases. Liver biopsy was not routinely performed to evaluate underlying liver disease. The FLR was reassessed by CT scan 4–6 weeks after PVE, just before surgery. The liver hypertrophy was defined using the following ratio: (FLR after PVE − FLR pre-PVE) × 100/(FLR pre-PVE). The persistence of a FLR volume of <30 % (normal parenchyma) or <40 % (in other cases) 2 months after PVE was considered a contraindication to resection.
Surgical procedures
Abdominal incision consisted of a xipho-supraumbilical laparotomy prolonged to the right subcostal area. Resection of 3 or more liver segments was considered a major hepatectomy. Abdominal exploration and intraoperative ultrasound were used to determine resectability. Transection of the hepatic parenchyma was performed by a combination of ultrasonic dissector and/or harmonic scalpel and wet bipolar forceps. Intermittent Pringle maneuver was used on demand during liver transection to control intraoperative blood loss. Lymphadenectomy was performed routinely and consisted of removal of all lymph nodes and connective tissue in the hepatoduodenal ligament and the retroduodenal area. Resection of I liver segment was always performed. Both distal and proximal margins of the sectioned bile ducts were sent for frozen section examination; presence of neoplastic cells in examined sections constituted an indication for widening of margins within the hepatic parenchyma as far as compatible with the feasibility of even multiple segmental biliary enteric anastomosis. Roux-en-Y biliary enteric reconstruction was performed using a segment of jejunum: transanastomotic stenting was not performed routinely. Vascular reconstructions of portal vein or hepatic artery were never preoperatively planned, but performed when required and technically feasible to reach R0 resections in patients with preoperatively unrecognized vessel involvement.
Outcome evaluation
For each patient data regarding preoperative evaluation and staging were recorded, as well as data about liver optimization and related events. Intraoperative data and postoperative outcome were evaluated, including blood losses and transfusion rate, length of postoperative stay, morbidity, and mortality. Postoperative complications were reviewed for 90 days following liver resection and were graded retrospectively according to Dindo–Clavien classification of surgical complications [10]. Postoperative mortality was defined as any death during postoperative hospitalization or within 90 days after resection. Three- and 5-year overall and disease-free survivals were evaluated using Kaplan–Meier method.
Statistical analysis
Demographic, pathological, operative details, and surgical outcomes were analyzed in the whole series and outcomes were compared between patients undergoing optimization and patients without optimization using the χ2 test or Fisher’s exact test for categorical data and the Mann–Whitney U test for ordinal data. Survival curves were generated using the Kaplan–Meier method. All data were expressed as mean plus the standard deviation or median and range. Significance was defined as P < 0.05. All analyses were performed using the statistical package SPSS 18.0 (SPSS, Chicago, IL).
Results
Between January 2004 and March 2012, 94 patients with hilar cholangiocarcinoma were admitted to Hepatobiliary Surgery Unit of San Raffaele Hospital and were candidates to surgical program after preliminary evaluation trough radiological imaging. Fifty patients (53.2 %) were females and 44 were males (46.8 %), with a median age of 59 (range 36–82) years. Preoperative tumor staging according to Bismuth–Corlette classification was the following: 5 patients (6.2 %) had type I, 29 patients (36.3 %) had type II, and 46 patients (57.5 %) had type III (respectively 27 type IIIa and 19 type IIIb).
Staging laparoscopy
Staging laparoscopy was performed in all the patients and successfully completed in 89 patients (94.7 %); in 5 cases it was not successful because of the presence of visceral adherences resulting from previous surgery (unrelated to hilar cholangiocarcinoma). Six patients (6.4 %) were excluded from surgery after staging laparoscopy demonstrated liver (2 cases) or peritoneal metastases (4 cases), with size below the detection limit of preoperative imaging. None of the patients was excluded from surgery because of the identification of locally advanced hilar disease at laparoscopic ultrasound during staging laparoscopy. Eight patients underwent laparotomy and could not benefit from surgical treatment because of advanced hilar disease (5 cases) or peritoneal metastases (3 cases). All of the patients who were excluded from surgery at laparotomy had been evaluated previously through staging laparoscopy and, in particular, two patients with advanced hilar disease and two with peritoneal metastases at laparotomy had undergone staging laparoscopy at a median of 22 days (range 10–34) before open exploration; in the other cases, staging laparoscopy was performed at the time of surgery as a preliminary step before laparotomy. None of the patients who did not receive staging laparoscopy were judged unresectable at laparotomy.
Biliary drainage
Fifty-five patients (58.5 %) presented with obstructive jaundice or radiological evidence of bile ducts dilatation and therefore required biliary drainage. In all patients, FLR was drained as a first choice. PTBD was external in 33 patients (60 %), external–internal in 13 (23.6 %), and endoscopic in 4 patients (7.3 %). Five patients (9.1 %) required placement of PTBD both in the FLR and in the liver to be resected, because serum total bilirubin level did not decreased adequately after first drain placement. Median interval between PTBD placement and surgery was 24 days (range 10–36). Bile replacement was never performed after PTBD. Serum total bilirubin level decreased from 12.7 ± 6.5 to 3.4 ± 1.5 mg/dL. Procedure-associated mortality was nil and morbidity rate was 18.2 %; ten patients developed complications, including one major event (hemorrhagic shock due to hemobilia, treated by angiographic embolization of the bleeding vessel) and nine minor complications (3 cases of drain dislodgement requiring its repositioning, 4 cases of cholangitis, 1 case of subcutaneous abscess, and 1 case of persistent pain with scarce response to analgesics).
Portal vein embolization
Fourteen patients (14.9 % of the whole series) had an inadequate FLR volume at preoperative evaluation and therefore were subjected to portal vein embolization (PVE). Twelve of these patients (85.7 %) also underwent preoperative PTBD. Mean FLR volume before PVE was 421.4 ± 151.3 cc and 33 ± 9.6 % of the total liver volume (TLV); after a median interval of 31 days (range 24–41), the mean FLR volume was 601.6 ± 161.7 cc and 39.5 ± 9.7 % of TLV, with a mean increase of 46.3 ± 30.4 % (P = 0.032). Procedure-related mortality was nil and morbidity was 14.3 %: one patient developed mild transaminases and leucocytes increase and one had leukocytosis with fever and diarrhea without sonographic signs of common portal vein thrombosis. Both complications were classified as minor and resolved after few days of hospital stay.
Surgical and histopathological data
Eighty patients (85.1 %) of 94 candidates to surgery underwent resection with curative intent. Five patients (6.2 %) underwent minor liver resection intending removal of segment IVb and V with biliary confluence resection. Sixty patients (75 %) underwent major hepatic resection, including 35 patients (43.7 %) undergoing right and 25 patients (31.2 %) undergoing left hepatectomy. Extended resections were performed in 15 patients (18.8 %), including 11 right hepatectomies (13.7 %) extended to segment IV and 4 left hepatectomies (5.1 %) extended to segment V and VIII. Caudate lobe resection was routinely performed. Lymphadenectomy was performed in all the patients, as previously described. Frozen sections from the proximal stumps of the biliary ducts turned positive in 39 patients (48.7 %), indicating the need for biliary resection widening to reach R0 resection. In 11 cases (13.7 %), a negative biliary stump was not achieved (R1 margins) due to the microscopic tumor infiltration above the technical limit for performing even multiple segmental biliary enteric anastomoses. Portal vein resection with end to end anastomosis was not routinely performed but carried out on a demand basis in 6 cases of vein wall infiltration by neoplastic tissue. Arterial resection and reconstruction were required in 4 patients showing encasement or infiltration of the vessel.
Histopathological data are summarized in Table 1. Fifty patients (62.5 %) had nodal involvement, whereas 30 patients were N0. R0 resection was achieved in 54 patients (67.5 %). Twenty-four patients (30 %) had R1 (11 because of biliary residual disease: 8 because of residual disease infiltrating the artery, 4 because of portal vein involvement, and 3 because of liver parenchyma infiltration). Two patients had macroscopic residual disease (both of them, undergoing left hepatectomy, had unresectable disease close to right hepatic artery) and were classified as R2.
Postoperative outcome
Forty-one patients developed postoperative complications, accounting for an overall morbidity of 51.2 %. Mortality was 6.2 %. In the same period, liver surgery-related morbidity in the whole series from our institution was 19.8 % and mortality was 1.5 % (respectively P = 0.035 and P = 0.044 compared with morbidity and mortality for hilar cholangiocarcinomas). Statistical significant differences were recorded even comparing morbidity/mortality rates associated with hilar cholangiocarcinoma surgery and morbidity/mortality associated with major liver resections for any other disease in our institution (respectively 25.4 %, P = 0.49 and 2.1 %, P = 0.47). Indeed, hilar cholangiocarcinoma surgery accounts for 3.5 % of the entire series of liver resections at our institution but is associated with 33.5 % of all mortalities.
Postoperative complications are summarized in Table 2. Most frequent complications were liver failure (10 patients, 12.5 %) and sepsis (10 patients, 12.5 %). All patients with postoperative liver failure and seven of ten patients with postoperative fever or sepsis developed other complications in the postoperative period. Overall, 41 patients (complications (class I and II according to Dindo–Clavien) and 21 major complications (class III and IV according to Dindo–Clavien) were recorded. Fifteen patients (18.7 %) developed more than one complication.
Five patients died in the postoperative period: three developed irreversible and progressive signs of liver failure, one had septic shock from cholangitis, and one developed massive pulmonary embolism leading to cardiac arrest. Median postoperative stay was 16 days (range 9–69).
Postoperative outcome and preoperative optimization
Fifty-five patients (71.2 %) received preoperative liver optimization (Fig. 2). Twenty-three patients, belonging to the first 2 years of the study, did not receive preoperative optimization program because this strategy had not entered our clinical practice yet. Comparison between preoperative optimization group (PreOp Group) and no preoperative optimization group (No PreOp Group) demonstrated that groups did not differ in terms of demographic data (age, sex, BMI), associated diseases, type of operation (distribution of left, right, extended resections). Patients in the PreOp Group had instead an higher incidence of advanced disease, both at preoperative (36 patients with type III in PreOp Group and 10 in No PreOp Group) and at histopathological evaluation (34 patients with T2 in PreOp Group and 14 in No PreOp Group; 15 patients with T3 in PreOp Group and 4 in No PreOp Group), even though not reaching statistically significant difference.
Table 3 shows comparison between groups in terms of postoperative morbidity, demonstrating a lower incidence of complications (45.6 %) in the PreOp Group compared with the No PreOp Group (65.2 %), mainly related to a low occurrence of infectious events (intrahepatic abscesses and sepsis) in the PreOp Group.
Mortality was not different between the groups (3 patients belonging to PreOp Group and 2 belonging to No PreOp Group died during the postoperative period).
Long-term survival
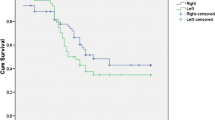
Median follow up was 19 months (range 3–94). The 3- and 5-year survival rate was respectively 43.5 % ± 9.4 and 39.5 % ± 8.7 months. Disease-free survival was 30.4 and 26.5 % at 3 and 5 years respectively. At univariate analysis, factors associated with prognosis were tumor grading, nodes status, and resection margins. Long-term outcomes were not different when comparing the PreOp Group to the Non PreOP group.
Discussion
Bile duct resection combined with major hepatectomy represents the standard of care for patients affected by hilar cholangiocarcinoma, because it allows obtaining a higher rate of R0 resections compared with bile duct resection alone or resection of IVb–V segments [2]. Better long-term survival experienced in past decades is the result of both increased rate of R0 resections and improved preoperative management and surgical technique. A radical resection with negative margins is the only factor with a demonstrated impact on outcome, on which the surgeon can have an influence and therefore should be the main goal of treatment [3].
Nevertheless, complex biliary and hepatic resections performed to achieve radical surgery are associated with significant risk of morbidity and mortality [3]. Series in the literature report morbidity rates ranging from 14 to 76 % and mortality rates between 0 and 19 % [3, 11–13]. The most life-threatening complications are represented by postoperative liver failure, primarily related to the extent of liver resection and to the presence of underlying parenchymal impairment, and by infectious complications, including cholangitis, liver and intra-abdominal abscesses, and wound infections, accounting for 50–80 % of all complications.
Capussotti et al. [14] reported that the occurrence of sepsis in patients with liver dysfunction contributed significantly to surgical mortality and therefore the treatment of any condition contributing to liver dysfunction is itself a treatment of liver failure and a factor potentially reducing mortality. In patients with Klatskin tumor, adequate preoperative management of candidates to surgery has been proposed as a factor that influences postoperative outcome, as well as a target to be aggressively reached through a multidisciplinary approach involving endoscopists, interventional radiologists, medical oncologists, and surgeons [15].
In the present series, the flowchart represented in Fig. 1 was used and a preoperative optimization program was based on few but crucial steps, including staging laparoscopy, biliary drainage, and possibly portal vein embolization. Staging laparoscopy is an additional tool to select patients suitable for curative surgery while extensive application of PTBD and PVE may to pave the way for a reduction of intraoperative and postoperative complications. Hirano et al. [16] reported that aggressive resection for hilar cholangiocarcinoma, performed in accordance with strict management strategy, achieved acceptably low mortality. These findings were confirmed in a study by Grandadam et al. [17] who stated that preoperative optimization reduces intra-abdominal septic complications, even though without impact on long-term survival.
Cytological or histological diagnoses are not always achievable in these patients before surgery because frequently tumoral mass is too small to allow percutaneous biopsy and brushing of the biliary tree is associated with significant incidence of false-negative results. However, the presence of benign biliary strictures is reported in the literature (incidence 15 %) [18], and patients should be aware of this possibility; despite this, it is recommended that if imaging studies demonstrate a focal stenosis in the absence of previous biliary tract surgery, a presumptive diagnosis of hilar cholangiocarcinoma is made until proven otherwise [15].
To avoid useless laparotomies, staging laparoscopy has been proposed as a standard procedure for patients with hilar cholangiocarcinoma with an accuracy of 42–53 % and a yield of 25–42 %, increased by 17 % thanks to the introduction of intraoperative ultrasounds [19, 20]. In the present series, the role of staging laparoscopy was confirmed in detection of peritoneal or hepatic localizations of disease with size below detection limit of imaging techniques, whereas it was reliable to rule out infiltration or encasement of hilar structures by tumoral tissue. Indeed, five patients required laparotomy to be excluded from treatment despite previous laparoscopy. Gaujoux [21] demonstrated how staging laparoscopy is less accurate in hilar cholangiocarcinoma compared with gallbladder cancer, because it lacks reliability in hilar resectability evaluation. The relatively low rate of unresectability (14.9 %) in the present series compared with other past series (40–50 %) may rely on a more accurate preoperative imaging, allowing an early detection of patients who are unfit for surgery [3, 22].
Recently, Gumbs et al. [23] reported a series that included five cases of totally laparoscopic resection of hilar cholangiocarcinoma, demonstrating feasibility and efficacy of minimal invasive techniques even in the treatment of liver tumors with hilar involvement. Nevertheless, role and indications to laparoscopy, apart from staging, have to be validated on a larger scale yet and require an extensive knowledge and experience both in hepatic and laparoscopic surgery to obtain radical surgery and thus acceptable morbidity and long-term benefit. Indeed, the main issues to deal with regard hilar structures dissection, adequate lymphadenectomy, and biliary reconstruction. In an attempt to reduce invasiveness of hilar cholangiocarcinoma surgery, robotic approach may be an option to be investigated even if, to our knowledge, no specific report in the literature exists at the moment.
Whether or not biliary tree drainage allows reduction in surgical morbidity and mortality is still controversial and also the strategy (endoscopic or radiological) for jaundice relief is a matter of debate in the literature [24–28]. Indeed, liver surgery in jaundiced patients is supposed to carry particular risks because of hepatic and systemic changes caused by hyperbilirubinemia, even though a systematic review from Liu et al. [24] did not provide evidence for a clinical benefit of using biliary drainage in jaundiced patients. Anyway, in the same review, the need for PVE is an absolute indication for drainage because jaundice is a recognized factor negatively affecting liver regeneration and hypertrophy. A more favorable outcome of percutaneous transhepatic biliary drainage compared with endoscopic stenting was reported in a recent study [25], maybe related to a higher incidence of infections and number of required procedures for endoscopic treatment. Furthermore, percutaneous drainage is more direct and effective in biliary tree decompression [26, 27].
In the present series, indication for biliary drainage placement consisted of obstructive jaundice or imaging evidence of bile ducts dilatation. The risk-benefit analyses considered on one hand benefits on patients symptoms (e.g., itch), hypertrophy of future remnant liver, and coagulopathy, together with a reduction of liver and kidney disfunction; on the other hand, it took into account drawbacks as intraoperative more complex evaluation of longitudinal extension of tumor, the need to delay surgery until blood tests normalization, and a significant risk of cholangitis. In particular, Ferrero et al. reported high risk of septic complication, regardless the type and the strategy of drainage [28].
In the present experience, patients undergoing preoperative optimization showed reduced postoperative morbidity in presence of an acceptable rate of complications related to optimization and a high rate of success in hyperbilirubinemia resolution, so that authors still recommend placement of percutaneous drainage of future liver remnant with a strong indication in cases of planned PVE because of an inadequate FLR, borderline volume of FLR, even in the absence of planned PVE; occurrence of complications secondary to chronic cholestasis (i.e., cholangitis, liver function worsening, malnutrition). In a series from Kennedy et al. [29], the true impact of preoperative drainage on postoperative outcome emerged only stratifying patients according to FLR volume. Indeed, an adequate FLR, even in presence of chronic cholestasis, seemed to possess enough functional reserve to tolerate extended resections, whereas marginal FLR requires biliary tree decompression to partially recover from functional impairment.
The role and indications to PVE have been deeply investigated by several studies from the literature [4, 30–32]. Nagino et al. [30] specifically addressed the topic of PVE in extrahepatic tumors, stating that the mortality rate was similar in patients who underwent extended hepatectomy following PVE and those who underwent resection of <50 % of the liver volume without PVE, clearly indicating that portal vein occlusion has potentially evident clinical benefits in patients undergoing extended liver volume resections. In a previous study from our group [31], as well as in this series, it is outstanding how PVE can significantly improve postoperative course of patients affected by hilar cholangiocarcinoma, who need large parenchymal sacrifice to obtain curative resection because of hilar structures involvements despite a small tumor volume. PVE increases FLR volume, restoring hepatic functional reserve, and therefore, lowering the risk of postoperative liver failure and allows surgery with curative intent in patients otherwise unresectable because of small FLR. Patients potentially resectable with normal liver functional should be candidates for PVE if FLR <25 %, whereas potentially resectable patients with impaired liver function (cholestasis, cirrhosis) should undergo PVE if FLR <40 %.
In summary, postoperative morbidity and mortality associated with hilar cholangiocarcinoma surgery is still high, especially compared with liver resections performed for other reasons. Staging laparoscopy has a role to rule out hepatic metastases or carcinomatosis undetected at imaging workup. Percutaneous biliary drainage placement is mandatory in candidates for PVE but also have may advantages in other subsets of patients. PVE contributes to lower the risk of liver failure. An adequate preoperative management (preoperative optimization) is therefore a factor that affects outcome of patients with Klatskin tumor, allowing acceptable morbidity and mortality rates in this still high-risk surgery.
References
Klatskin G (1965) Adenocarcinoma of the hepatic duct at its bifurcation within the porta hepatis. An unusual tumor with distinctive clinical and pathological features. Am J Med 38:241–256
Ito F, Agni R, Rettammel RJ et al (2008) Resection of hilar cholangiocarcinoma: concomitant liver resection decreases hepatic recurrence. Ann Surg 248:273–279
Ito F, Cho CS, Rikkers LF et al (2009) Hilar cholangiocarcinoma: current management. Ann Surg 250(2):210–218
Abdalla EK, Barnett CC, Doherty D et al (2002) Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg 137(6):675–680
Shoup M, Gonen M, D’Angelica M et al (2003) Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg 7(3):325–330
Vauthey JN, Chaoui A, Do KA et al (2000) Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical association. Surgery 127(5):512–519
Bismuth H, Corlette MB (1975) Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet 140(2):170–178
American Joint Committee on Cancer (1997) Extrahepatic bile ducts. In: Fleming ID, Cooper JS et al (eds) AJCC cancer staging manual. Lippincott-Raven, Philadelphia, pp 109–111
Jarnagin WR, Fong Y, DeMatteo RP et al (2001) Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg 234(4):507–517
Clavien PA, Barkun J, de Oliveira ML et al (2009) The Clavien–Dindo classification of surgical complications: five-years experience. Ann Surg 250(2):187–196
Nimura Y, Kamiya J, Kondo S et al (2000) Aggressive preoperative management and extended surgery for hilar cholangiocarcinoma: Nagoya experience. J Hepatobiliary Pancreat Surg 7:155–162
Todoroki T, Kawamoto T, Koike N et al (2000) Radical resection of hilar bile duct carcinoma and predictors of survival. Br J Surg 87:306–313
Jarnagin WR, Shoup M (2004) Surgical management of cholangiocarcinoma. Semin Liver Dis 24:189–199
Capussotti L, Viganò L, Giuliante F et al (2009) Liver dysfunction and sepsis determine operative mortality after liver resection. Br J Surg 96(1):88–94
Van Gulik TM, Kloek JJ, Ruys AT et al (2001) Multidisciplinary management of hilar cholangiocarcinoma (Klatskin tumor): extended resection is associated with improved survival. Eur J Surg Oncol 37(1):65–71
Hirano S, Kondo S, Tanaka E et al (2010) Outcome of surgical treatment of hilar cholangiocarcinoma: a special reference to postoperative morbidity and mortality. J Hepatobiliary Pancreat Sci 17(4):455–462
Grandadam S, Compagnon P, Arnaud A et al (2010) Role of preoperative optimization of the liver for resection in patients with hilar cholangiocarcinoma type III. Ann Surg Oncol 17(12):3155–3161
Erdogan D, Kloek JJ, ten Kate FJ et al (2008) Immunoglobulin G4-related sclerosing cholangitis in patients resected for presumed malignant bile duct strictures. Br J Surg 95(6):727–734
Weber SM, DeMatteo RP, Fong Y et al (2002) Staging laparoscopy in patients with extrahepatic biliary carcinoma. Analysis of 100 patients. Ann Surg 235(3):392–399
Goere D, Wagholikar GD, Pessaux P et al (2006) Utility of staging laparoscopy in subsets of biliary cancers: laparoscopy is a powerful diagnostic tool in patients with intrahepatic and gallbladder carcinoma. Surg Endosc 20(5):721–725
Gaujoux S, Allen PJ (2010) Role of staging laparoscopy in peri-pancreatic and hepatobiliary malignancy. World J Gastrointest Surg 2(9):283–290
Ruys AT, Busch OR, Gouma DJ et al (2011) Staging laparoscopy for hilar cholangiocarcinoma: is it still worthwhile? Ann Surg Oncol 18(9):2647–2653
Gumbs AA, Jarufe N, Gayet B (2013) Minimally invasive approaches to extrahepatic cholangiocarcinoma. Surg Endosc 27(2):406–414
Liu F, Li Y, Wei Y et al (2011) Preoperative biliary drainage before resection for hilar cholangiocarcinoma: whether or not? A systematic review. Dig Dis Sci 56(3):663–672
Kloek JJ, van der Gaag NA, Aziz Y et al (2010) Endoscopic and percutaneous preoperative biliary drainage in patients with suspected hilar cholangiocarcinoma. J Gastrointest Surg 14(1):119–125
Nimura Y, Hayakawa N, Kamiya J et al (1990) Hepatic segmentectomy with caudate lobe resection for bile duct carcinoma of the hepatic hilus. World J Surg 14(4):535–543. doi:10.1007/BF01658686
Bismuth H, Nakache R, Diamond T (1992) Management strategies in resection for hilar cholangiocarcinoma. Ann Surg 215(1):31–38
Ferrero A, Lo Tesoriere R, Viganò L et al (2009) Preoperative biliary drainage increases infectious complications after hepatectomy for proximal bile duct tumor obstruction. World J Surg 33(2):318–325. doi:10.1007/s00268-008-9830-3
Kennedy TJ, Yopp A, Qui Y et al (2009) Role of preoperative biliary drainage of liver remnant prior to extended liver resection for hilar cholangiocarcinoma. HPB (Oxford) 11(5):445–451
Nagino M, Kamiya J, Nishio H et al (2006) Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg 243(3):364–372
Ratti F, Soldati C, Catena M et al (2010) Role of portal vein embolization in liver surgery: single centre experience in sixty-two patients. Updates Surg 62(3–4):153–159
Sano T, Shimada K, Sakamoto Y et al (2006) One hundred two consecutive hepatobiliary resections for perihilar cholangiocarcinoma with zero mortality. Ann Surg 244(2):240–247
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ratti, F., Cipriani, F., Ferla, F. et al. Hilar Cholangiocarcinoma: Preoperative Liver Optimization with Multidisciplinary Approach. Toward a Better Outcome. World J Surg 37, 1388–1396 (2013). https://doi.org/10.1007/s00268-013-1980-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-013-1980-2