Abstract
Purpose
Recurrent laryngeal nerve (RLN) palsy is the major concern of reoperative thyroid surgery, and the introduction of neuromonitoring could reduce the rate of this complication. The present study is a retrospective analysis of the experience with completion thyroidectomy with and without neuromonitoring in a referral center.
Methods
Between October 1999 and April 2011, 246 patients [37 men, 209 women; mean age, 55 ± 12.5 (range, 25–80) years] underwent 250 reoperations for recurrent goiter (n = 203), hyperthyroidism (n = 26), or recurrent thyroid cancer (n = 17). The mean interval between the initial and the reoperative procedure was 17.5 years. According to the availability of the neuromonitoring system and to the surgeon preference, 91 operations were performed with neuromonitoring (NM-group), whereas 159 were performed with direct nerve visualization (NV-group) alone. Patients’ characteristics, perioperative data, and postoperative complications were collected in a prospectively maintained database.
Results
In the NM-group, 51 unilateral and 40 bilateral resections were performed. The NV-group included 122 unilateral and 37 bilateral procedures. The number of nerves at risk after previous surgery was 128 (NM-group) and 161 (NV-group), respectively. We registered eight RLN palsy in the NM-group (6.2 %) and four in the NV-group (2.5 %; p = 0.1).
Conclusions
The routine use of intraoperative neuromonitoring seems not to reduce the incidence of RLN during redo thyroid surgery, at least in the setting of a tertiary referral center.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reoperations represent a major challenge in thyroid surgery, because they are associated with an increased complications rate. The incidence of definitive recurrent laryngeal nerve (RLN) palsy and permanent hypoparathyroidism has been reported to range between 0 and 3.1 and 0 and 2.9 %, respectively [1–10]. Beside the difficulty of nerve visualization, dissection of scar tissue surrounding the recurrent laryngeal nerve or the vascular pedicle of the parathyroid glands can increase the risk of postoperative complications. Especially RLN injury affects the quality of life, resulting in a wide spectrum of symptoms ranging from voice changes to even severe airway obstruction in case of bilateral palsy [11] and is the major cause of litigation in endocrine surgery [12]. Nerve visualization is the best prevention against its inadvertent damage [13] and has represented the standard in thyroid surgery for more than three decades [14]. The concept of intraoperative RLN stimulation has been developed during the past two decades and recommendations for its clinical use have been recently standardized [15]. Despite increasing acceptance among experienced endocrine surgeons [16], the accuracy of intraoperative neuromonitoring (ioNM) for predicting RLN palsy is affected by several pitfalls that account for a relatively low sensitivity and positive predictive value [17]. Nevertheless, neuromonitoring allows for control of the nerve function during surgery and also aids nerve localization before identification. Latter characteristic makes this tool extremely attractive for application during redo thyroid surgery.
The present study was performed to review the extent and type of complications occurring during secondary thyroidectomy, focusing on the impact of intraoperative neuromonitoring compared with a control group operated on by relying on nerve visualization only.
Patients and methods
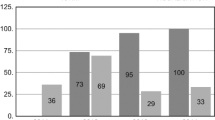
Between November 1999 and April 2011, 4,489 thyroid operations were performed on 4,392 patients (3,298 women, 1,094 men) with a mean age of 50 ± 14 (range, 3–90) years. A total of 246 patients [37 men, 209 women; mean age, 55 ± 12.5 (range, 25–80) years] underwent 250 reoperations for recurrent goiter (n = 203), hyperthyroidism (n = 26), or recurrent thyroid cancer (n = 17). Intraoperative neuromonitoring was used in 89 patients (78 female, 11 male; mean age: 55 ± 12 years) operated between 2003 and 2011 (NM-group). One hundred fifty-seven patients (131 female, 26 male; mean age, 55 ± 13 years) operated on without nerve monitoring served as the control group (NV-group). Patients’ characteristics, preoperative diagnosis, and the distribution between the groups during the overall study period are summarized in Table 1 and Fig. 1, respectively. The mean interval between the initial and the reoperative procedure was 17.5 (range, 3–57) years. Excluded from this study were patients with completion thyroidectomy for cancer. Moreover, patients with unilateral surgery on the previously not resected side, and patients with recurrence of the pyramidal lobe were not included in the calculation of risk of postoperative RLN palsy. Two hundred operations were performed by two surgeons with extensive experience in thyroid surgery (each with more than 1,000 thyroidectomies performed), whereas the remaining 50 resections by 6 surgeons with moderate experience (more than 100 thyroidectomies performed).
Patients scheduled for thyroid surgery did not receive a preoperative sedation. General anesthesia was induced intravenously with propofol, remifentanil, and the short-acting muscle relaxant, mivacurium. Subsequently, it was maintained as balanced anesthesia with isoflurane and remifentanil.
Neck ultrasound was routinely performed by the surgeon himself, preoperatively. Thereby, the extent of the recurrence and the presence of the isthmus were analyzed. A lateral approach was performed if the ultrasound showed adhesions between strap muscle and trachea due to the absence of the thyroid isthmus. In case of a recurrence involving the isthmic region, a medial approach between the strap muscles was preferred. A standard cervicotomy with excision of the existing scar was performed in all patients except ten, for which a minimally invasive video-assisted approach was used. In case of bilateral recurrence, the procedure was started on the larger lobe. The first step was ligation of the superior thyroid vessels (if still present) with special attention to the preservation of the superior laryngeal nerve and upper parathyroid gland. Then, the RLN was clearly identified and dissected carefully along its course. Because of the previous adhesions, special care was made to identify the lower parathyroid gland near to the thyroid lobe and a fine dissection was performed to preserve the vascular pedicle.
Among 250 reoperations, there were 66 total thyroidectomies, 7 subtotal thyroidectomies, 152 lobectomies, 22 subtotal lobectomies/nodule resections, and 3 lymph nodes dissection in the central compartment because of carcinoma (Table 2). According to the extent of previous surgery and type of recurrence, the number of nerves at risk has been identified in 128 (NM-group) and 161 (NV-group), respectively. A parathyroid autotransplantation in the sternocleidomastoid or sternothyroid muscle was performed in 16 patients (6.5 %). Suction drainage was used in 144 procedures (58 %).
Intraoperative neuromonitoring was used by all participating surgeons according to the availability and to the personal preference. The Neurosign® (InoMed, Teningen, Germany) was used until 2009. The system allows direct nerve stimulation with a needle-electrode inserted into the vocal muscle through the cricothyroid ligament. The neutral electrode is placed into the sternocleidomastoid muscle. Since 2010, it was replaced by the NIM 3.0 Nerve Monitoring System® (Medtronic, Jacksonville, FL), which was used with either endotracheal surface electrode or external needle-electrode. The correct position of the endotracheal tube electrode was confirmed by video laryngoscopy and not checked during the surgical procedure, as neck hyperextension was avoided in both groups. The current amplitude was set between 0.5 and 1 mA. Nerve function was tested in all cases after identification and dissection by means of both direct and vagal stimulation. A predissection vagal stimulation as routine procedure was introduced in 2010. Moreover, ioNM allowed in selected cases for delimiting the area of dissection by stimulation of the scar tissue supposed to trap the nerve. Experience with the neuromonitoring system was gained during the overall study period on more than 500 thyroid and parathyroid procedures. For the present analysis, a loss of signal during surgery was considered true positive (TP) if confirmed by a postoperative laryngoscopy showing RLN palsy. A loss of signal with a normal postoperative vocal cords assessment was interpreted as false positive (FP). False negative (FN) was considered an intact intraoperative signal followed by postoperative palsy and true negative (TN) an intact signal followed by normal laryngoscopy. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were calculated according to Chan and Lo [18].
Direct laryngoscopy was performed routinely before surgery and on the first postoperative day. Blood samples for calcium were obtained postoperatively in case of symptoms. Symptomatic hypocalcaemia was defined as a Ca++ <2 mmol/l with clinical manifestations, such as tingling or numbness of the extremity or carpo-pedal spasm. The incidence of nerve palsy was calculated based on the number of nerves at risk. Recurrent laryngeal nerve palsy was considered permanent if there was no recovery 6 months after surgery. Hypoparathyroidism was considered permanent if the patient requires calcium and/or vitamin D supplement to maintain normal serum calcium level for 6 months or longer.
Patients’ characteristics, perioperative data, as well as postoperative complications were collected in a prospectively maintained database. Follow-up was performed by contacting the patients or their general physician.
Statistical analysis
Data are expressed as mean (range) or mean ± SD as appropriate. Group differences at different time points were examined by the Mann–Whitney test; categorical data were analyzed by the Fisher’s exact test. Statistical significance was set at p < 0.05. Data were analyzed using GraphPad Prism 5® (GraphPad Software, Inc.).
Results
There was no perioperative mortality in both groups. Of a total of 289 nerves at risk in both groups, the overall incidence of postoperative transient nerve palsy was 4.1 % and it was not significantly different between the groups (p = 0.1). One patient in the NV-group with a preoperative unilateral RLN palsy showed a bilateral RNL palsy necessitating tracheostomy for airway control. Ten unilateral nerve palsies were reversible (one patient was lost to follow-up). The incidence of hemorrhages as well as hypoparathyroidism was not significantly influenced by the use of the neuromonitoring system. Postoperative complications are summarized in Table 3.
The overall sensitivity, specificity, PPV, NPV, and accuracy of ioNM were 37.5, 95, 43, 94, and 88 %, respectively. In two patients, malposition of the endotracheal surface electrode was supposed, because vagal stimulation before starting the dissection was not possible. Among the eight patients with a postoperative RLN palsy in the NM-group, five showed a false-negative signal by the direct nerve stimulation and three a false-negative signal by the vagus stimulation. The use of intraoperative neuromonitoring was associated with increased operating time for both unilateral and bilateral procedures, even if not statistically significant (Table 4). Final histology showed a thyroid carcinoma in 8 patients (9 %) in the NM-group and in 11 cases in the NV-group (7 %). The mean postoperative hospital stay was 2 days in both groups. The patient who required tracheostomy had a definitive RLN palsy and died 4 years later from causes unrelated to thyroid surgery. During follow-up, a second patient died because of metastasized follicular thyroid cancer. After a mean follow-up of 4.5 ± 2.9 years (range, 3 months–11 years), no patient required another thyroid surgery.
Discussion
Secondary thyroidectomy has become an uncommon operation due to the wide acceptance of total thyroidectomy as standard treatment for multinodular goiter [19]. Available data obtained through a review of the literature are summarized in Table 5. Moreover, most of the published series include completion thyroidectomy after lobectomy for differentiated thyroid carcinoma and contralateral recurrent goiter after previous hemithyroidectomy. It is questionable whether those situations should be compared to a reoperation for recurrent goiter. In most cases, the second procedure consists on a contralateral lobectomy in an operative field not explored during the first operation and therefore without any local scar. For the present study, scrupulous review of the description of the operation has been performed and true recurrences could be separately analyzed. Of particular concern is the finding of a high incidence of vocal cord palsy after the initial procedure, many of which had been unsuspected by normal phonation. For this reason, we strongly recommend a routine preoperative investigation of the vocal cord function. This procedure is standard in our unit for any patient scheduled for a thyroid operation. All in all, 289 “nerves at risk” were identified with an overall incidence of postoperative nerve palsy of 4.1 %. This rate is higher than most of the series that report on primary thyroidectomy [19]. Nevertheless, all of one recovered within 6 months (rate of permanent nerve palsy: 0.3 %).
In a large multi-institutional study of 16.448 operations, the incidence of permanent RLN palsy for 1,480 benign recurrent goiter and 124 recurrent thyroid malignancy was reported to be 3.6 and 5.1 %, respectively [20]. The large number of participating centers with variable experience in thyroid surgery affects the results of this study. Nevertheless, analysis of those results strength the conviction that reoperative thyroid surgery should be selectively performed in centers with high experience in thyroid surgery. Despite any effort made to ensure nerve functionality, the fact is that redo thyroid surgery is associated with the highest risk of postoperative palsy. Intraoperative neuromonitoring might reduce this risk by facilitating nerve visualization into the scar tissue. In a prospective, randomized study of 1,000 patients (1,000 nerves at risk for each group), the prevalence of transient RLN palsy was significantly lower in the group of patients who had surgery with ioNM by 2.9 % in high-risk patients and 0.9 % in low-risk patients [21]. Despite those encouraging results, the rate of permanent nerve palsy was not reduced by the use of intraoperative neuromonitoring compared with visual nerve identification only [20, 21]. The results of many prospective nonrandomized studies comparing nerve visualization with (19,290 nerves at risk) or without (6,671 nerves at risk) adjunct of intraoperative neuromonitoring support this conclusion [17]. RLN palsy rate (both transient and permanent) tended to be lower with intraoperative neuromonitoring, but the difference was not statistically significant. Beside the questionable advantage of this tool, the relatively low sensitivity and positive predictive value seems to further discourage its routine use.
The present study is to our knowledge the largest comparative analysis to evaluate the results of intraoperative neuromonitoring in recurrent thyroid diseases. The decision to use ioNM was made preoperatively according to the availability of the instrument and surgeon’s preference adapted to the extent of the surgical procedure. This explains the higher number of bilateral total thyroidectomy in the NM-group. Certainly, the decision to adopt the ioNM also could have been influenced by the preoperative assessment of the risk of the procedure, but the higher incidence of RLN palsy in the NM-group seems not be justified by a supposed major difficulty of the procedure in this group of patients. Moreover, all operation reports have been reviewed, and if a scar in the region of the nerve was not described the patients were excluded from the calculation of risk of postoperative nerve palsy. The Mayo Clinic group reported similar results, comparing 52 cervical re-explorations performed under continuous electromyographic monitoring to 59 nonmonitored operations [22]. Due to the different patient population (mostly suffering from recurrent thyroid cancer), the incidence of RLN palsy was higher compared with our study but not different between the two groups. The logical explanation for those results is, in our opinion, related to the special feature of the operation. Beside nerve identification, its dissection from the surrounding scar tissue represents in many cases the most difficult step of the procedure, which cannot be influenced by the actually available intermittent nerve monitoring systems. Moreover, nerve stretching recognized as one of the main mechanisms for nerve injury is in some cases unavoidable to dissect the region of Barry’s ligament [23]. The introduction of continuous nerve monitoring could represent a promising innovation to avoid dissection-related nerve injuries [24]. At the present time, the only advantage of ioNM for redo thyroid surgery is to reconsider the surgical strategy if loss of signal is registered after completed dissection of the first site in patients with bilateral disease to prevent bilateral RLN palsy [25]. This was the case in two patients in the present series. In one patient, the finding of intraoperative neuromonitoring was confirmed by postoperative laryngoscopy (transient palsy reversible after 3 months); in the second case, the result of ioNM was false positive. Both patients have been operated on a second time (4 and 2 months after previous surgery) to remove the contralateral lobe. If ioNM is used, both RLN and vagus nerve stimulation are mandatory. This was confirmed in the present study by the false-negative results of RLN stimulation in five of eight patients with a postoperative palsy.
Extensive experience in thyroid surgery should be still considered the most important factor for approaching redo thyroid surgery. To minimize the risk of RLN palsy, we suggest starting the dissection of the dorsal aspect of the thyroid in the region with theoretically less scar tissue. Generally it is possible to visualize the nerve into the upper mediastinum or between larynx and thyroid lobe if the upper thyroid vessels have not been divided during the first procedure. Moreover, the practice of leaving a small amount of thyroid tissue in the region of the ligament of Berry should be endorsed to prevent nerve stretching and minimize the risk of injury in this critical area.
In conclusion, ioNM cannot take the place of routine nerve visualization and careful dissection, which remain crucial to avoid postoperative complications.
References
Reeve TS, Delbrige L, Brady P et al (1988) Secondary thyroidectomy: a 20-year experience. World J Surg 12:449–453. doi:10.1007/BF01655417
Levin KE, Clark AH, Duh QY et al (1992) Reoperative thyroid surgery. Surgery 111:604–609
Chao TC, Jeng LB, Lin JD et al (1997) Reoperative thyroid surgery. World J Surg 21:644–647. doi:10.1007/s002689900287
Peix JL, Van Box Som P, Olagne E et al (1997) Results of reoperation for goiter. Ann Chir 51:217–221
Makeieff M, Rubinstein P, Youssef B et al (1998) Repeat surgery for thyroid nodules (excluding cancer and hyperthyroidism). Ann Chir 52:970–977
Wilson DB, Staren ED, Prinz RA (1998) Thyroid reoperations: indications and risks. Am Surg 64:674–679
Menegaux F, Turpin G, Dahman M et al (1999) Secondary thyroidectomy in patients with prior thyroid surgery for benign disease: a study of 203 cases. Surgery 125:479–483
Müller PE, Jakoby R, Heinert G et al (2001) Surgery for recurrent goitre: its complications and their risk factors. Eur J Surg 167:816–821
Gibelin H, Sierra M, Mothes D et al (2004) Risk factor for recurrent nodular goiter after thyroidectomy for benign disease: case-control study of 224 patients. World J Surg 28:1079–1082. doi:10.1007/s00268-004-7607-x
Lefevre JH, Tresallet C, Leenhardt L et al (2007) Reoperative surgery for thyroid disease. Langenbeck Arch Surg 392:685–691
Smith E, Taylor M, Mendoza M et al (1998) Spasmodic dysphonia and vocal fold paralysis: outcomes of voice problems on work-related functioning. J Voice 12:223–232
Abadin SS, Kaplan EL, Angelos P (2010) Malpractice litigation after thyroid surgery: the role of recurrent laryngeal nerve injuries, 1989–2009. Surgery 148:718–723
Lahey FH, Hoover WB (1938) Injuries to the recurrent laryngeal nerve in thyroid operations: their management and avoidance. Ann Surg 108:545–562
Jatzko GR, Lisborg PH, Müller MG et al (1994) Recurrent nerve palsy after thyroid operations: principal nerve identification and a literature review. Surgery 115:139–144
Randolph GW, Dralle H, The International Intraoperative Monitoring Study Group (2011) Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope 121(Suppl 1):S1–S16
Sturgeon C, Sturgeon T, Angelos P (2009) Neuromonitoring in thyroid surgery: attitudes, usage patterns, and predictors of use among endocrine surgeons. World J Surg 33:417–425. doi:10.1007/s00268-008-9724-4
Dralle H, Sekulla C, Lorenz K et al (2008) Intraoperative monitoring of the recurrent laryngeal nerve in thyroid surgery. World J Surg 32:1358–1366. doi:10.1007/s00268-008-9483-2
Chan WF, Lo CY (2006) Pitfalls of intraoperative neuromonitoring for predicting postoperative recurrent laryngeal nerve function during thyroidectomy. World J Surg 30:182–806. doi:10.1007/s00268-005-0355-8
Moalem J, Suh I, Duh QY (2008) Treatment and prevention of recurrence of multinodular goiter: an evidence-based review of the literature. World J Surg 32:1301–1312. doi:10.1007/s00268-008-9477-0
Dralle H, Sekulla C, Haerting J et al (2004) Risk factors of paralysis and functional outcome after recurrent laryngeal nerve monitoring in thyroid surgery. Surgery 136:1310–1322
Barczyski M, Konturek A, Cichoń S (2009) Randomized clinical trial of visualization versus neuromonitoring of recurrent laryngeal nerves during thyroidectomy. Br J Surg 96:240–246
Yarbrough DE, Thompson GB, Kasperbauer JL et al (2004) Intraoperative electromyographic monitoring of the recurrent laryngeal nerve in reoperative thyroid and parathyroid surgery. Surgery 136:1107–1115
Chiang FY, Lu IC, Kuo WR et al (2008) The mechanism of recurrent laryngeal nerve injury during thyroid surgery: the application of intraoperative neuromonitoring. Surgery 143:743–749
Ulmer C, Koch KP, Seimer A et al (2008) Real-time monitoring of the recurrent laryngeal nerve: an observational clinical trial. Surgery 143:359–565
Goretzki PE, Schwarz K, Brinkmann J et al (2008) The impact of intraoperative neuromonitoring (IONM) on surgical strategy in bilateral thyroid diseases: is it worth the effort? World J Surg 34:1274–1284. doi:10.1007/s00268-009-0353-3
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alesina, P.F., Rolfs, T., Hommeltenberg, S. et al. Intraoperative Neuromonitoring does not Reduce the Incidence of Recurrent Laryngeal Nerve Palsy in Thyroid Reoperations: Results of a Retrospective Comparative Analysis. World J Surg 36, 1348–1353 (2012). https://doi.org/10.1007/s00268-012-1548-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-012-1548-6